Anouk Frieling MSc Equine Sciences, BSc (Hons)
Vitamin E is a lipid-soluble antioxidant, synthesised by plants through photosynthesis (Reboul, 2017), that has multifunctional purposes in the body (Rigotti, 2007; Traber & Head, 2021). It was discovered in 1922 during a study in rats where it was identified as an important element for reproductive processes in the body (Traber, 2007). Further research throughout the years discovered the other important functions Vitamin E has in the body, such as supporting the immune system, liver function and muscle health (Finno & Valberg, 2012). It was also suggested that Vitamin E deficiencies are related to various health issues and diseases in the horse (Finno and Valberg, 2012). Therefore, this article highlights the importance of Vitamin E in the horse’s diet by explaining the different types of Vitamin E and the different functions it has in the body.
VITAMIN E STRUCTURE
Vitamin E is a term used for eight molecules with a similar structure, four tocopherols and four tocotrienols (Schneider, 2005). Tocopherols consist of one chromanol ring and a saturated phytyl tail whereas tocotrienols consist of a chromanol ring and an unsaturated isoprenyl tail with three double bonds (Lee & Han, 2018; Niki & Abe, 2019) (Figure 1). These forms of Vitamin E are designated into α, β, ƴ and δ depending on the location and number of the methyl group (CH3) on the chromanol ring (Niki & Abe, 2019). For example, the α-form of tocopherol and tocotrienol has two methyl groups on R1, R2 and R3 (Figure 1) (Brigelius-Flohé, 2006; Niki & Abe, 2019). Each of the subgroups (α, β, ƴ and δ both in tocopherols and tocotrienols) exist in eight different forms known as stereoisomers, with the α-form of tocopherol being the form of Vitamin E we are most interested in from a nutritional point of view. As such, this article will focus on α-tocopherol.
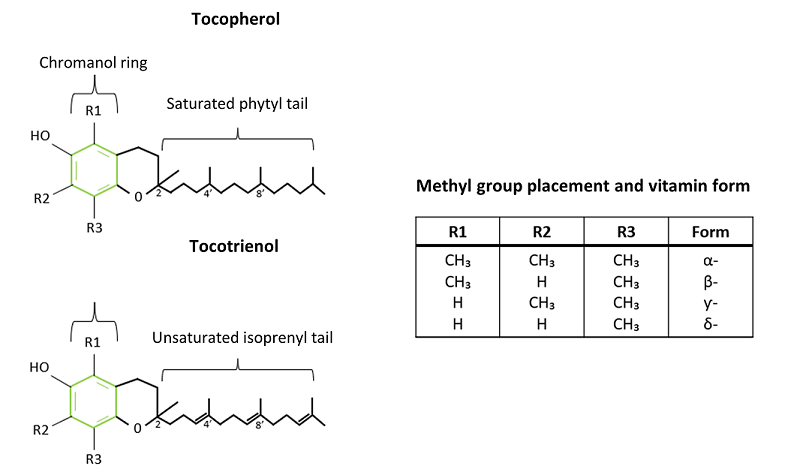
Figure 1. The structures of the two different types of vitamin E, tocopherol and tocotrienol. The green hexagon displays the structure of the chromanol ring. A saturated phytyl tail is attached to the chromanol ring of the tocopherol and an unsaturated isoprenyl tail is attached to the tocotrienol chromanol ring. The table includes the different placements and amounts of methyl groups which translates in the different tocopherol and tocotrienol forms. (Adapted from Lee & Han, 2018)
Tocopherols contain three chiral carbons: one on C2 of the chromanol ring and two on the tail at C4 and C8 (Figure 2) (Niki & Abe, 2019). Each of these chiral carbons can be configured in either the R configuration or S configuration. This means that there are eight possible configurations of tocopherols (RRR, RRS, RSS, RSR, SSS, SSR, SRR and SRS), with each configuration representing the R or S configuration of each of the three chiral carbons (Vagni et al., 2011).
The tocopherol configuration is important as enzymes and proteins within the body have evolved to work specifically with certain configurations. The RRR configuration is produced naturally in plants, therefore the most natural and abundant form of Vitamin E is RRR-α-tocopherol (Herrera & Barbas, 2001; Niki & Abe, 2019).
NATURAL AND SYNTHETIC VITAMIN E
Vitamin supplements can consist of two types of Vitamin E, natural or synthetic Vitamin E (Finno & Valberg, 2012). Synthetic Vitamin E contains equal proportions of all eight stereoisomers and therefore contains both R or S configurations (Vagni et al., 2011). This means that synthetic Vitamin E contains 1/8th or 12.5%, natural RRR-α-tocopherol. There are two types of synthetic Vitamin E, all-rac-α-tocopherol and dl-α-tocopherol (Finno & Valberg, 2012), with all-rac-α-tocopherol acetate being the most common form used in supplements (Vagni et al., 2011). Previous human and animal studies, including horses (Fiorellino et al., 2009; Fagan et al., 2020), have shown that the body favours the natural variant of Vitamin E (RRR form) over the synthetic form of Vitamin E (Lauridsen et al., 2002; Lodge, 2005; Cheng et al., 2016), therefore the bioavailability of the synthetic Vitamin E is lower in comparison to the natural form (Vagni et al., 2011). This means that a larger amount of natural Vitamin E is absorbed by the body in comparison to synthetic Vitamin E, even if both types of Vitamin E are presented in similar amounts in the diet (Cheng et al., 2016). Therefore, natural Vitamin E is suggested to have more effect on the body and is also more efficient than synthetic Vitamin E as the body is more likely to absorb and transport a higher concentration of natural Vitamin E (Clemente et al.,2015).
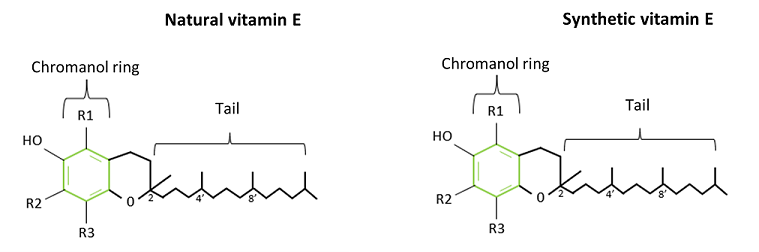
Figure 2. Natural vitamin E and synthetic vitamin E structure showing configuration R on 2', 4' and 8' of natural vitamin E and R or S configuration on the structure of synthetic vitamin E.
VITAMIN E DIGESTION AND ABSORPTION
It is suggested that the digestion of Vitamin E starts in the duodenum, part of the small intestine, as the stomach is thought to have no significant effect on Vitamin E digestion (Borel et al., 2001). In the duodenum digestive enzymes such as proteases, amylase and lipases mix with the food and release Vitamin E (Borel et al., 2013). Vitamin E that is not already incorporated into vegetable oils is transferred into micelles in combination with dietary fats (Rich et al., 2003; Borel et al., 2013). These micelles are thought to be essential for Vitamin E absorption (Reboul, 2017), which mainly takes place in the small intestine where it is suggested that α- and ƴ-tocopherols are absorbed in similar amounts, depending on the concentration of the Vitamin E forms in the diet (Rigotti, 2007). After absorption the Vitamin E is then transported to the liver where it is stored for further use (Rigotti, 2007). When Vitamin E is required by the body it is secreted into the circulation from the liver, bound to a very-low-density lipoprotein (VLDL), which is required to transport the Vitamin E to different target cells (Di Donato et al., 2010). Even though α- and ƴ-tocopherols are absorbed in similar amounts, in rat studies RRR-α-tocopherol was found to be more frequently transported through the body after absorption in comparison to other Vitamin E forms, suggesting that the natural form of Vitamin E is the favoured form by the body (Ikeda et al., 1996). Natural Vitamin E is most likely favoured by the body due to α-tocopherol transfer protein (α-TTP), a liver protein, which binds Vitamin E to VLDLs (Di Donato et al., 2010). It is suggested that α-TTP selectively favours α-tocopherols in comparison to other Vitamin E forms (Di Donato et al., 2010). Based on the body’s response to different Vitamin E forms, horses requiring higher levels of Vitamin E, such as those with muscle, liver or immunity issues, are likely to benefit from being fed natural Vitamin E.
FUNCTIONS OF VITAMIN E
An imbalance of production and accumulation of oxygen reactive species (ROS) can create free ranging free radicals in the body (Pizzino et al., 2017). A free radical is a molecule or atom that has an odd number of electrons and therefore the atom or molecule becomes instable (Phaniendra et al., 2015). To become stable the free radicals react with biochemicals in the body to gain electrons to create an even number, therefore the biochemical becomes a free radical as it then has an odd number of electrons (Phaniendra et al., 2015). This chain reaction can create damaging oxidative stress to cells and tissues in different parts of the body (Pizzino et al., 2017; Garcia et al., 2022). The main function of the antioxidant Vitamin E is to bind to these free radicals and neutralise them to prevent them from creating oxidative stress (Fagan et al., 2020). Oxidative stress can for example affect the liver, which has many functions within the body, including the storage and transportation of Vitamin E (Singal et al., 2011). Therefore, Vitamin E supplementation can offer support to animals with liver diseases due to oxidative stress (Bansal et al., 2005; Singal et al., 2011; Tallon & McGovern, 2020). The Vitamin E requirement of a healthy adult horse fed equivalent to 2% of their body weight per day as fibre, is between 1 and 2IU per day/kg body weight depending on the horse’s workload (NRC, 2007). It is recommended to feed two to three times this amount to horses affected by liver issues as this amount is likely to be beneficial (Carr & Holcombe, 2009).
Free radicals are able to damage immune cells since they are prone to oxidative damage (Coquette et al., 1986; Meydani et al., 2005). Studies in animals and humans have shown that when the daily Vitamin E intake is increased that this has a significant effect on the immune response (Meydani et al., 1990; Han & Meydani, 1999). For example, a study in horses demonstrated a connection between an increased immune response, measured through an increase of the antibody Immunoglobin G (IgG), and an increased supplementation of Vitamin E or a combination of Vitamin E and Selenium after horses were given a tetanus or equine influenza vaccination (Baalsrud & Øvernes, 1986). Another study showed that administration of 2500IU of RRR-α-tocopherol per day to pregnant mares increased the antibody concentration of IgG and IgM in colostrum which resulted in a higher IgM concentration in blood plasma of the foals (Bondo & Jensen, 2011). This increased concentration supports the foal’s immune system and improves protection against infectious diseases after birth (Bondo & Jensen, 2011). Therefore, it is suggested that Vitamin E enhances immunity and increases pathogen resistance in the body (Meydani et al., 2005).
As mentioned previously, Vitamin E was discovered due to its effects on reproductive processes in the rat (Traber, 2007). Oxidative stress can also affect reproductive processes in horses, such as oocyte maturation, spermatozoa function and pregnancy, and can therefore cause infertility in mares and stallions (Finno & Valberg, 2012). The effects of Vitamin E supplementation on stallion fertility has been analysed in previous studies, showing a significant positive effect of supplementation of 3000IU Vitamin E per day on the fertility of stallions (Gee et al., 2008). For more information about the effects of Vitamin E supplementation on stallion fertility please refer to Nutrition of the stallion. It is clear that a Vitamin E deficiency can result in infertility in males and females (Rengaraj & Hong, 2015), which is why supplementation is suggested for breeding stock that receives a diet that does not provide the required daily Vitamin E.
Oxidative stress is suggested to increase during heavy exercise which can have an effect on the muscles as it can result in muscle tissue damage (Kinnunen et al., 2005). Because of the multiple functions of Vitamin E, it is suggested that Vitamin E supplementation can have an effect on oxidative stress in heavily exercised horses and will reduce the risk of muscle damage (Williams & Carlucci, 2006; Fagan et al., 2020). Research investigating the effects of Vitamin E on oxidative stress in horses performing exercise has shown contrasting results. McMeniman & Hintz (1992) and Williams et al. (2004) were unable to identify significant effects after Vitamin E supplementation on oxidative stress post heavy exercise, whereas more recent research suggested that Vitamin E has the potential to decrease oxidative stress in heavily exercised horses (Fagan et al., 2020). Fagan et al. (2020) fed adult horses, performing heavy exercise and weighing approximately 550kg, 4000IU Vitamin E per day. This is considerably higher than current NRC (2007) recommendations of 1000IU Vitamin E per day for a 500kg horse performing such exercise, showing the importance of continued research into equine nutrition and updating of recommendations.
VITAMIN E DEFICIENCY AND HEALTH ISSUES
In horses there are diseases related to consistent Vitamin E deficiency in the body; tying-up, polysaccharide storage myopathy (PSSM), equine motor neuron disease (EMND), equine degenerative myeloencephalopathy (EDM) and Vitamin E deficient myopathy (Finno & Valberg, 2012). Tying-up, also called rhabdomyolysis, is a disease which affects the muscles and can occur sporadically or chronically (Tozaki et al., 2010). The chronic form of tying-up is called polysaccharide storage myopathy (PSSM) (Valberg, 2014). Tying-up can be caused due to respiratory infections, exhaustive and excessive training, or it can be caused due to a lack of Vitamin E and Selenium in the horses diet (Valberg, 2014). Signs of tying-up are muscle stiffness, discomfort when moving, muscle contractions such as muscle spasm in the hindquarters, and an elevated heart rate (Bouwman et al., 2010).
Polysaccharide storage myopathy (PSSM) is a muscle disorder and was first recognised in Quarter Horses (Valberg et al., 1999). This disease causes abnormal polysaccharide concentrations in the skeletal muscle fibres of the horse and accumulation of glycogen (Nollet & Deprez, 2005). Polysaccharide storage myopathy occurs in two variants, type I and type II (Valberg, 2014). Polysaccharide storage myopathy type I is mainly found in draft horses and Quarter Horse related breeds. Type I is connected to a mutation on the glycogen synthase gene (GYS1) in horses (Stanley et al., 2009) (Stanley et al., 2009), and therefore the disease can be diagnosed by performing a genetic test. The clinical symptoms are related to rhabdomyolysis (tying-up) and occur when the horse performs exercise (Firshman et al., 2005). If the horse starts showing clinical symptoms related to PSSM regularly the horse owner is advised not to exercise the horse for an extended period, however due to the genetic mutation clinical signs will reappear as soon as the horse starts exercising again (Firshman et al., 2005). Horses with PSSM type II show the same muscle glycogen abnormalities and the accompanying clinical signs but lack the mutation on GYS1, and therefore the specific cause of PSSM type II is not known (Williams et al., 2018). In comparison to type I, type II mainly occurs in Warmblood and Thoroughbred horses (Valberg, 2014). It is advised that the diet of horses with PSSM or tying-up is supplemented with additional fat as it is suggested to act beneficially for horses with this disease. Because of the high-fat diet it is suggested to supplement the diet with 600 to 6000IU Vitamin E per day to support muscle health (Mckenzie et al., 2002; Williams, 2008).

Figure 3. The large muscles of the back and hindquarters of horses that are affected by a muscle disorder show signs of weakness or cramping. These clinical signs can be supported by feeding additional dietary Vitamin E.
Equine motor neuron disease (EMND) is a disease affecting the somatic lower motor neurons and ventral horns of the spinal cord and selected brain stem nuclei, caused by prolonged and consistent Vitamin E deficiency (Cummings et al., 1990). This disease can only be diagnosed post-mortem but clinical signs are weight loss due to muscle wasting, muscle twitching and horses lying down for prolonged periods (Divers et al., 1994). The affected neurons are suppliers of type I muscle fibres which are responsible for slow muscle contractions and are highly resistant against fatigue (Finno & Valberg, 2012; Ciciliot et al., 2013). Therefore, the muscle fibre supply is also affected by EMND (Finno & Valberg, 2012). This disease is mainly found in horses that have no access to pasture and/or fresh forage, as fresh pasture is normally the main Vitamin E supplier in the horse’s diet (Divers et al., 1994). Therefore, it is recommended to supplement Vitamin E to horses which are not able to graze on pasture (Finno & Valberg, 2012) and if EMND is suspected it is suggested to supplement the feed of a 500kg horse with 5000IU per day for a prolonged period (Naylor, 2014).
Equine degenerative myeloencephalopathy (EDM) is a genetic neurodegenerative disease related to Vitamin E deficiencies (Burns & Finno, 2018). One of the most significant clinical signs is development of ataxia (uncoordinated movement) during the first year of the horse’s life (Burns & Finno, 2018). Other clinical signs are that the horse seems to overshoot where they place their limbs (hypermetria) when being walked with an elevated head, and an abnormal stance at rest. Some affected horses also seem to have a decreased fight or flight response (Aleman et al., 2011; Burns & Finno, 2018). It is suggested that the disease is related to a decreased level of α-tocopherol in the first four months of a horse’s life (Burns & Finno, 2018). This disease cannot be treated but the supplementation of Vitamin E is suggested to decrease the severity of the symptoms (Finno & Valberg, 2012; Burns & Finno, 2018).
SUMMARY
Vitamin E is an antioxidant with multiple functions in the body and is therefore an important vitamin in the horse’s diet. Natural Vitamin E (RRR-α-tocopherol) is the most abundant Vitamin E form and is also the most bioavailable form of Vitamin E. Horses mainly derive Vitamin E from fresh forage from pasture, therefore it is advised to supplement Vitamin E to horses if they have little to no access to pasture. Equine Vitamin E supplements either contain natural or synthetic Vitamin E, which have similar structures but have different configurations, with natural Vitamin E having greater bioavailability in comparison to synthetic Vitamin E. Natural Vitamin E has greater bioavailability as the body favours this form over other Vitamin E forms and therefore, natural Vitamin E is absorbed in higher concentrations. The main function of Vitamin E is to neutralise free radicals in the body which are created due to oxidative stress. Increased Vitamin E supplementation is also known to support liver function, muscle tissue, reproductive functions and increased immune responses to prevent diseases and infections. The Vitamin E requirements of a healthy horse are between 1 and 2IU per day/kg body weight, which can differ depending on workload. It is recommended to increase the daily natural Vitamin E intake by two to three times to support horses with liver issues. A study has shown that to support the immune system Vitamin E intake can be increased to 2500IU/day and to support fertility it can be increased to 3000IU/day. Horses that are affected by muscle issues or that perform heavy exercise and therefore need muscle health support can benefit from an increased Vitamin E intake up to 4000IU per day. Prolonged Vitamin E deficiencies can cause disorders such as tying-up, equine motor neuron disease and equine degenerative myeloencephalopathy. Polysaccharide storage myopathy is also a muscle disease and a form of tying-up that can be related to a genetic mutation. Increased Vitamin E supplementation is beneficial for horses that are affected by these disorders. In conclusion, Vitamin E is an important nutrient in the horse’s diet, and it is therefore important to meet the daily requirements by providing a tailored diet. For extra support the daily amount can be increased by providing a (natural) Vitamin E supplement.
REFERENCES
Aleman, M., Finno, C. J., Higgins, R. J., Puschner, B., Gericota, B., Gohil, K., LeCouteur, R. A. & Madigan, J. E. (2011) Evaluation of epidemiological, clinical, and pathological features of neuroaxonal dystrophy in Quarter Horses. Journal of the American Veterinary Medical Association, 239(6): 823-833.
Baalsrud, K. J. & Øvernes, G. (1986) Influence of vitamin E and selenium supplement on antibody production in horses. Equine Veterinary Journal, 18(6): 472-474.
Bansal, A. K., Bansal, M., Soni, G. & Bhatnagar, D. (2005) Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chemico-Biological Interactions, 156(2–3): 101-111.
Bondo, T. & Jensen, S. K. (2011) Administration of RRR-α-tocopherol to pregnant mares stimulates maternal IgG and IgM production in colostrum and enhances vitamin E and IgM status in foals. Journal of Animal Physiology and Animal Nutrition, 95(2): 214-222.
Borel, P., Pasquier, B., Armand, M., Tyssandier, V., Grolier, P., Alexandre-Gouabau, M. C., Andre, M., Senft, M., Peyrot, J., Jaussan, V., Lairon, D. & Azais-Braesco, V. (2001) Processing of vitamin A and E in the human gastrointestinal tract. American Journal of Physiology - Gastrointestinal and Liver Physiology, 280(1 43-1): 96-103.
Borel, P., Preveraud, D. & Desmarchelier, C. (2013) Bioavailability of vitamin E in humans: An update. Nutrition Reviews, 71(6): 319–331.
Bouwman, F. G., Van Ginneken, M. M. E., Van Der Kolk, J. H., Van Breda, E. & Mariman, E. C. M. (2010) Novel markers for tying-up in horses by proteomics analysis of equine muscle biopsies. Comparative Biochemistry and Physiology - Part D: Genomics and Proteomics, 5(2): 178-183.
Brigelius-Flohé, R. (2006) Bioactivity of vitamin E. Nutrition Research Reviews, 19: 174-186.
Burns, E. N. & Finno, C. J. (2018) Equine degenerative myeloencephalopathy: prevalence, impact, and management. Veterinary Medicine: Research and Reports, 9: 63-67.
Carr, E. A. & Holcombe, S. J. (2009) Nutrition of Critically Ill Horses. Veterinary Clinics of North America - Equine Practice, 25(1): 93-108.
Cheng, K., Niu, Y., Zheng, X. C., Zhang, H., Chen, Y. P., Zhang, M., Huang, X. X., Zhang, L. L., Zhou, Y. M. & Wang, T. (2016) A comparison of natural (D-α-tocopherol) and synthetic (DL-α-tocopherol acetate) Vitamin E supplementation on the growth performance, meat quality and oxidative status of broilers. Asian-Australasian Journal of Animal Sciences, 29(5): 681-688.
Ciciliot, S., Rossi, A. C., Dyar, K. A., Blaauw, B. & Schiaffino, S. (2013) Muscle type and fiber type specificity in muscle wasting. International Journal of Biochemistry and Cell Biology, 45(10): 2191-2199.
Clemente, H. A., Ramalho, H. M. M., Lima, M. S. R., Grilo, E. C. & Dimenstein, R. (2015) Maternal supplementation with natural or synthetic vitamin e and its levels in human colostrum. Journal of Pediatric Gastroenterology and Nutrition, 60(4): 533-537.
Coquette, A., Vray, B. & Vanderpas, J. (1986) Role of vitamin E in the protection of the resident macrophage membrane against oxidative damage. Archives Internationales de Physiologie et de Biochimie, 94(5): 29-34.
Cummings, J. F., de Lahunta, A., George, C., Fuhrer, L., Valentine, B. A., Cooper, B. J., Summers, B. A., Huxtable, C. R. & Mohammed, H. O. (1990) Equine motor neuron disease; a preliminary report. The Cornell veterinarian, 80(4): 357-379.
Divers, T. J., Mohammed, H. O., Cummings, J. F., Valentine, B. A., De Lahunta, A., Jackson, C. A. & Summers, B. A. (1994) Equine motor neuron disease: findings in 28 horses and proposal of a pathophysiological mechanism for the disease. Equine Veterinary Journal, 26(5): 409-415.
Di Donato, I., Bianchi, S. & Federico, A. (2010) Ataxia with vitamin e deficiency: Update of molecular diagnosis. Neurological Sciences, 31(4): 511-515.
Fagan, M. M., Harris, P., Adams, A., Pazdro, R., Krotky, A., Call, J. & Duberstein, K. J. (2020) Form of Vitamin E Supplementation Affects Oxidative and Inflammatory Response in Exercising Horses. Journal of Equine Veterinary Science, 91: 1-12.
Finno, C. J. & Valberg, S. J. (2012) A Comparative Review of Vitamin E and Associated Equine Disorders. Journal of Veterinary Internal Medicine, 26: 1251-1266.
Fiorellino, N. M., Lamprecht, E. D. & Williams, C. A. (2009) Absorption of Different Oral Formulations of Natural Vitamin E in Horses. Journal of Equine Veterinary Science, 29(2): 100-104.
Firshman, A. M., Baird, J. D. & Valberg, S. J. (2005) Prevalences and clinical signs of polysaccharide storage myopathy and shivers in Belgian Draft Horses. Journal of the American Veterinary Medical Association, 227(12): 1958-1964.
Garcia, E. I. C., Elghandour, M. M. M. Y., Khusro, A., Alcala-Canto, Y., Tirado-González, D. N., Barbabosa-Pliego, A. & Salem, A. Z. M. (2022) Dietary Supplements of Vitamins E, C, and β-Carotene to Reduce Oxidative Stress in Horses: An Overview. Journal of Equine Veterinary Science, 110: 1-6.
Gee, E. K., Bruemmer, J. E., Siciliano, P. D., McCue, P. M. & Squires, E. L. (2008) Effects of dietary vitamin E supplementation on spermatozoal quality in stallions with suboptimal post-thaw motility. Animal Reproduction Science, 107(3–4): 324-325.
Han, S. N. & Meydani, S. N. (1999) Vitamin E and infectious diseases in the aged. Proceedings of the Nutrition Society, 58(3): 679-705.
Herrera, E. & Barbas, C. (2001) Vitamin E: Action, metabolism and perspectives. Journal of Physiology and Biochemistry, 57: 43-56.
Ikeda, I., Imasato, Y., Sasaki, E. & Sugano, M. (1996) Lymphatic transport of alpha-, gamma- and delta-tocotrienols and alpha-tocopherol in rats. International Journal for Vitamin and Nutrition Research, 66(3): 217-21 .
Kinnunen, S., Hyyppä, S., Lappalainen, J., Oksala, N., Venojärvi, M., Nakao, C., Hänninen, O., Sen, C. K. & Atalay, M. (2005) Exercise-induced oxidative stress and muscle stress protein responses in trotters. European Journal of Applied Physiology, 93(4): 496-501.
Lauridsen, C., Engel, H., Jensen, S. K., Morrie Craig, A. & Traber, M. G. (2002) Lactating sows and suckling piglets preferentially incorporate RRR- over all-rac-α-tocopherol into milk, plasma and tissues. Journal of Nutrition, 132(6): 1258-1264.
Lee, G. Y. & Han, S. N. (2018) The role of vitamin E in immunity. Nutrients, 10(11): 1-18.
Lodge, J. K. (2005) Vitamin E bioavailability in humans. Journal of Plant Physiology, 162(7): 790-796.
Mckenzie, E. C., Valberg, S. J. & Pagan, J. D. (2002) A Review of Dietary Fat Supplementation in Horses with Exertional Rhabdomyolysis. AAEP Proceedings, 48: 381-386.
McMeniman, N. P. & Hintz, H. F. (1992) Effect of vitamin E status on lipid peroxidation in exercised horses. Equine Veterinary Journal, 24(6): 482-484.
Meydani, S. N., Barklund, M. P., Liu, S., Meydani, M., Miller, R. A., Cannon, J. G., Morrow, F. D., Rocklin, R. & Blumberg, J. B. (1990) Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. American Journal of Clinical Nutrition, 52(3): 557-563.
Meydani, S. N., Han, S. N. & Wu, D. (2005) Vitamin E and immune response in the aged: Molecular mechanisms and clinical implications. Immunological Reviews, 205: 269-284.
Naylor, R. (2014) Managing muscle disease in horses. In Practice, 36(8): 418-423.
Niki, E. & Abe, K. (2019) Chapter 1-Vitamin E: Structure, Properties and Functions. In Niki, E. Food Chemistry, Function and Analysis Vitamin E: Chemistry and Nutritional Benefits, 11th Ed. Royal Society Of Chemistry, London, UK.
Nollet, H. & Deprez, P. (2005) Hereditary skeletal muscle diseases in the horse. A review. Veterinary Quarterly, 27(2): 65-75.
NRC (2007) Nutrient Requirements of Horses, 6th Ed. The National Academies Press, Washinton, USA.
Phaniendra, A., Jestadi, D. B. & Periyasamy, L. (2015) Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian Journal of Clinical Biochemistry, 30(1): 11-26
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D. & Bitto, A. (2017) Oxidative Stress: Harms and Benefits for Human Health. Oxidative Medicine and Cellular Longevity, 2017: 1-13.
Reboul, E. (2017) Vitamin e bioavailability: Mechanisms of intestinal absorption in the spotlight. Antioxidants, 6(4): 1-11.
Rengaraj, D. & Hong, Y. H. (2015) Effects of dietary vitamin E on fertility functions in poultry species. International Journal of Molecular Sciences, 16(5): 9910-9921.
Rich, G. T., Faulks, R. M., Wickham, M. S. J. & Fillery-Travis, A. (2003) Solubilization of carotenoids from carrot juice and spinach in lipid phases: II. Modeling the duodenal environment. Lipids, 38(9): 947-956.
Rigotti, A. (2007) Absorption, transport, and tissue delivery of vitamin E. Molecular Aspects of Medicine, 28(5-6): 423-436.
Schneider, C. (2005) Chemistry and biology of vitamin E. Molecular Nutrition and Food Research, 49(1): 7-30.
Singal, A. K., Jampana, S. C. & Weinman, S. A. (2011) Antioxidants as therapeutic agents for liver disease. Liver International, 31(10): 1432-1448.
Stanley, R. L., McCue, M. E., Valberg, S. J., Mickelson, J. R., Mayhew, I. G., McGowan, C., Hahn, C. N., Patterson-Kane, J. C. & Piercy, R. J. (2009) A glycogen synthase 1 mutation associated with equine polysaccharide storage myopathy and exertional rhabdomyolysis occurs in a variety of UK breeds. Equine Veterinary Journal, 41(6): 597-601.
Tallon, R. & McGovern, K. (2020) Equine liver disease in the field. Part 2: causes and management. UK-Vet Equine, 4(3): 71-76.
Tozaki, T., Hirota, K., Sugita, S., Ishida, N., Miyake, T., Oki, H. & Hasegawa, T. (2010) A genome-wide scan for tying-up syndrome in Japanese Thoroughbreds. Animal Genetics, 41: 80-86.
Traber, M. G. (2007) Vitamin E regulatory mechanisms. Annual Review of Nutrition, 27: 347-362.
Traber, M. G. & Head, B. (2021) Vitamin E: How much is enough, too much and why. Free Radical Biology and Medicine, 177: 212-225.
Vagni, S., Saccone, F., Pinotti, L. & Baldi, A. (2011) Vitamin E Bioavailability: Past and Present Insights. Food and Nutrition Sciences, 2(10): 1088-1096.
Valberg, S. J. (2014) Exertional Myopathies in Horses. MSD Manual Veterinary Manual, 1: 1–3.
Valberg, S. J., Mickelson, J. R., Gallant, E. M., MacLeay, J. M., Lentz, L. & de la Corte, F. (1999) Exertional rhabdomyolysis in quarter horses and thoroughbreds: one syndrome, multiple aetiologies. Equine veterinary journal. Supplement, 30: 533-538.
Williams, C. A. & Carlucci, S. A. (2006) Oral vitamin E supplementation on oxidative stress, vitamin and antioxidant status in intensely exercised horses. Equine Veterinary Journal, 38(36): 617-621.
Williams, C. A., Kronfeld, D. S., Hess, T. M., Saker, K. E., Waldron, J. N., Crandell, K. M., Hoffman, R. M. & Harris, P. A. (2004) Antioxidant supplementation and subsequent oxidative stress of horses during an 80-km endurance race. Journal of Animal Science, 82(2): 588-594.
Williams, J. (2008) PSSM in the genes?. SA Horsemen: Health & Feeding, 3(4): 44–45.
Williams, Z. J., Bertels, M. & Valberg, S. J. (2018) Muscle glycogen concentrations and response to diet and exercise regimes in Warmblood horses with type 2 Polysaccharide Storage Myopathy. PLoS ONE, 13(9): 1-17.

