Tania Sundra
BSc(Hons) BVMS MANZCVS (Equine Medicine)
Avon Ridge Equine Veterinary Services, Brigadoon, WA, Australia
David Rendle
BVSc MVM CertEM(IntMed) DipECEIM FRCVS
RCVS and European Specialist in Equine Internal Medicine
EMT Consulting, Tiverton, Devon, UK
INTRODUCTION
The term equine gastric ulcer syndrome (EGUS) was previously used to describe squamous gastric disease and glandular gastric disease (Sykes et al., 2015a) (Figure 1). More recently however, there has been a growing appreciation of the differences between both these diseases with a realisation that the pathophysiology, risk factors, treatment and management of squamous disease cannot be extrapolated to glandular disease. Whilst both conditions may occur concurrently, it does not necessarily mean there is an association between them.
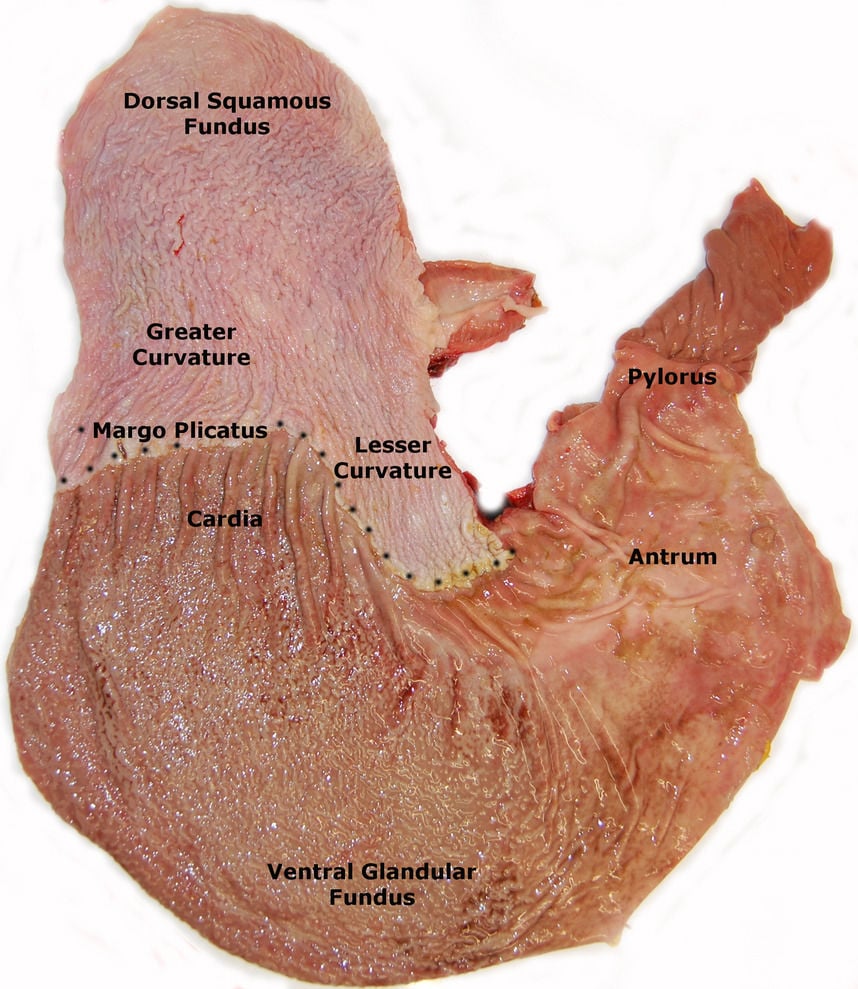
Figure 1. A postmortem specimen of the equine stomach depicting the anatomical regions of the stomach (Sykes et al., 2015).
Acid suppressive therapy using proton-pump inhibitors remains the cornerstone of treatment for both diseases. Oral omeprazole is the only proton-pump inhibitor registered for the treatment of squamous disease, however, it is no longer considered effective for the treatment of glandular disease (Sykes et al., 2015a; Rendle et al., 2018). Following recent trends in human medicine, other alternatives are being explored for both the management of glandular disease, and to improve healing rates for squamous disease. However, implementing management changes to reduce risk factors should be initiated alongside drugs to help improve healing rates and to prevent recurrence of disease when treatment is completed.
EQUINE SQUAMOUS GASTRIC DISEASE
RISK FACTORS AND MANAGEMENT
Squamous gastric disease primarily occurs as a result of acid injury to a tissue that has limited defence against a low pH environment. Any disruption to the normal stratification of gastric pH will therefore increase the risk of squamous disease. Excessive exposure to acid causes the stratified squamous epithelium to become thickened and hyperkeratotic. Continued acid exposure leads to sloughing of the superficial epithelium which can progress to deeper lesions and areas of erosion and ulceration (Martineau et al., 2009) (Figure 2).
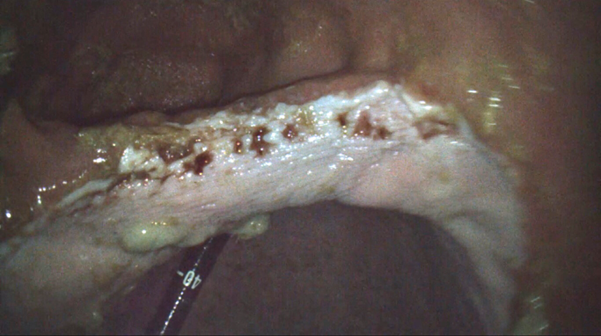
Figure 2. Grade 2 squamous disease on the lesser curvature of the stomach. Credit: T. Sundra.
Diet and exercise have consistently been shown to play a major role in the development of squamous disease (Murray & Eichorn, 1996; Vatistas et al., 1999; Begg & O’Sullivan, 2003; Bell et al., 2007; Luthersson et al., 2009). In horses prone to squamous disease, grain feeding should be eliminated from the diet to reduce the production of volatile fatty acids (Luthersson et al., 2009). For horses in heavy work, or those that require additional calories, oils provide a safer alternative to cereals. In a study conducted in ponies, corn oil supplementation (45ml/day for 5 weeks) was beneficial to gastric health and was shown to decrease gastric acid production and increase prostaglandin production (Cargile et al., 2004). Maize (corn), vegetable or canola oil can all be used at up to 1 ml/kg body weight per day. Oil should be gradually introduced over a few weeks to allow the horse’s metabolism to adapt. Horses should receive supplementation with vitamin E (2000 iu/day) when feeding oil, to mitigate the potential for oxidative injury due to increased free radical production.
Feed deprivation has also been shown to cause an increase in gastric acidity, a reduction in intraluminal pH and the development of squamous disease (Murray & Eichorn, 1996). A study conducted on racehorses in Australia demonstrated that those with free access to pasture were 3 times less likely to have squamous disease (Lester et al., 2008). A 2015 consensus statement (Sykes et al., 2015a) also recommended free access to good quality grass pasture or frequent feeding of hay (4-6 meals/day) to help restore the normal stratification of gastric pH. In one study, feeding straw as the sole roughage source increased the prevalence of squamous disease (Luthersson et al., 2009), however a recent study indicated that a 50% straw diet would not cause gastric ulcers to develop (Jansson et al., 2021).
Exercise causes gastric compression, disruption to the stratification of gastric pH and ‘splashing’ of acidic fluid onto the squamous mucosa (Lorenzo-Figueras & Merritt, 2002). Feeding a small amount (2-3L) of roughage prior to exercise to prevent this acid ‘splash’ would therefore seem logical to reduce the risk of squamous. As the duration of acid exposure also parallels the risk for development of squamous disease (Murray et al., 1996), horses prone to squamous disease should ideally be trained with short duration, high intensity exercise programs.
Environmental factors have also been demonstrated to play a role in the development of squamous disease. Horses are herd animals and direct contact with others appears to reduce the risk of squamous (Lester et al., 2008). A rural setting and access to pasture also reduce risk (Lester et al., 2008).
In cases where risk factors are persistent (e.g. racehorses in training), the prophylactic use of oral omeprazole should be considered. A 2019 meta-analysis demonstrated that omeprazole prevented squamous disease in horses in training with only 23% of treated horses developing squamous disease, compared to 77% which were given no omeprazole prophylaxis (Mason et al., 2019).
TREATMENT OPTIONS
Oral omeprazole is the only registered treatment for squamous disease and in early studies resulted in 70-77% healing of squamous lesions in racehorses in training (Murray et al., 1997; Andrews et al., 1999; Doucet et al., 2003; Lester et al., 2005). There has been a growing appreciation of the influence of diet and compliance on the efficacy of oral omeprazole (Sykes, 2019). The absorption of omeprazole is affected by feeding and administration on an empty stomach improves bioavailability (Sykes et al., 2015c). Omeprazole treatment should therefore be administered after an overnight fast, 1-2 hours prior to feeding.
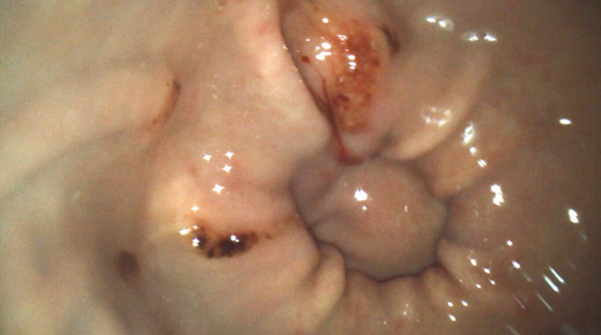
Figure 3. An example of equine glandular gastric disease lesions at the pylorus. Credit: T. Sundra.
Esomeprazole has attracted particular interest in human, and more recently equine medicine as it is absorbed more consistently and metabolised more slowly than omeprazole (Andersson et al., 2001). Compared to an equivalent dose of omeprazole, esomeprazole results in a more pronounced and consistent acid suppressive effects in man (Lind et al., 2000; Rohss et al., 2000; Rohss et al., 2001) and similar benefits have now been demonstrated in horses (Sykes et al., 2017b). Esomeprazole may therefore offer advantages over omeprazole and may be more effective for both treatment and prevention of squamous disease, particularly in racehorses where failure rates for treatment and prevention with omeprazole may be 30% and 20% respectively (Sykes et al., 2015a; Mason et al., 2019).
The highest rates of squamous healing are reported following the administration of an unregistered long-acting injectable omeprazole (LAIO) preparation. Intramuscular injection overcomes any issues with bioavailability or owner compliance with the administration of oral preparations. Administration of a LAIO preparation to 6 horses demonstrated the capacity of the injectable preparation to supress acid more profoundly and consistently than when the same horses were administered oral omeprazole every day (Sykes et al., 2017a). In the treatment of squamous disease in racehorses in Australia, 100% healing was reported with LAIO after only 2 injections one week apart (Sykes et al., 2017a). In a retrospective study performed in the UK, 93% healing of squamous lesions was observed with 4 weekly injections of LAIO with most cases having healed within 2 weeks after only 2 injections (Gough et al., 2020).
GLANDULAR DISEASE
RISK FACTORS AND MANAGEMENT
Unlike the squamous mucosa, the glandular epithelium has several mechanisms to protect it from injury by hydrochloric acid. The pathogenesis of glandular disease is poorly understood but it has been hypothesised that failure of normal defence mechanisms may predispose the glandular mucosa to injury (Rendle et al., 2018) (Figure 3). We now know that how we manage squamous disease cannot simply be applied to the management of glandular disease.
In recent years, a growing body of evidence has indicated that both physiological and psychological stress may play a role in the development of glandular disease (Malmkvist et al., 2012; Scheidegger et al., 2017; Pedersen et al., 2018; Sykes et al., 2019). Frequency of exercise has been demonstrated to be an important risk factor; the risk of glandular disease was shown to increase 10-fold in Thoroughbreds exercised more than 5 days per week (Sykes et al., 2019). Another study in Canadian showjumpers found a 5-fold increase in risk of disease in horses exercised more than 6 times per week (Pedersen et al., 2018). It has been postulated that the frequency of exercise may negatively impact gastric blood flow, thereby impairing the normal protective mechanisms of the gastric mucosa. There is evidence that limiting exercise to up to 5 days per week may reduce the risk of glandular disease (Rendle et al., 2018).
Trainer has been identified as a risk factor for the development of glandular disease in Thoroughbreds (Sykes et al., 2019) and in a riding horse population having a greater number of handlers and riders was associated with a greater risk of disease (Mönki et al., 2016). Measures to minimise stress should be tailored to each individual as the effectiveness of specific interventions will vary widely between horses.
Mucosal protectants such as pectin and lecithin complexes may be beneficial in the prevention of glandular disease (Sykes et al., 2013; Rendle et al., 2018). Lecithin is a phospholipid and pectins are highly viscous substances which may aid to stabilise the protective mucus layer (Sykes et al., 2013) of the glandular mucosa, however robust clinical trials to prove their efficacy are lacking.
TREATMENT OPTIONS
There are currently no medications registered for the treatment of glandular disease and oral omeprazole is considered ineffective (Rendle et al., 2018) with healing in less than 50% of cases being reported (Sykes et al., 2014). Whilst acid injury is not thought to initiate glandular, a low pH may perpetuate mucosal injury and prevent healing (Sykes et al., 2015a). As a result, acid suppression remains the cornerstone of therapy for glandular disease and a number of different options including, omeprazole and sucralfate combination, LAIO, esomeprazole or misoprostol are currently being utilised.
Oral omeprazole and sucralfate in combination has been reported to result in glandular healing rates of between 20-71% (Hepburn & Proudman, 2014; Varley et al., 2019; Barton, et al., 2021). In some populations the response to this combination is disappointing and the combination may also present practical challenges regarding the administration of both medications at different times on an empty stomach as the absorption of oral omeprazole may be affected both by feeding and the administration of sucralfate (Sykes et al., 2015c; Sykes, 2019)
Like squamous disease, the highest rates of healing of glandular disease have been reported following the use of LAIO (Sykes et al., 2017a; Gough et al., 2022). LAIO also overcomes compliance and bioavailability issues that is associated the administration of daily, oral medications (Sykes et al., 2015c; Rendle et al., 2018). After 2 weeks of treatment with LAIO, 9 of 12 horses were reported to have healed in a study of racing Thoroughbreds (Sykes et al., 2017a). In a retrospective study in the UK, rates of glandular disease healing after 4 weeks of treatment were 82% with LAIO compared to 50% with oral omeprazole (Gough et al., 2022)
Esomeprazole is being used with increasing frequency in equine practice as a first-line treatment for horses with glandular disease (Sundra, 2021) and to facilitate healing of both squamous and glandular lesions which failed to respond to oral omeprazole (Rendle, 2017).
Misoprostol has been reported to result in healing of glandular in 55-72% cases (Varley et al., 2019; Pickles et al., 2020), however in one report 42% of cases treated with misoprostol, squamous disease either developed or worsened (Pickles et al., 2020). There are significant human health and safety concerns with the use of misoprostol and it needs to be handled very carefully.
CONCLUSION
Optimising diet and management are fundamental to the prevention and management of gastric disease. Careful consideration should be given to the constituents of the diet, the timing and frequency of feeding, exercise routines and there should be a focus on minimising stressors. Acid-suppressive medications remain the cornerstone of therapy for both squamous and glandular disease. Whilst oral omeprazole has been proven effective for the management of squamous disease it is largely ineffective for the treatment of glandular disease. New (mostly unregistered) alternatives are being explored and show promise in improving healing rates of both conditions.
REFERENCES:
Andersson, T., Rohss, K., Bredberg, E. & Hassan-Alin, M. (2001). Pharmacokinetics and pharmacodynamics of esomeprazole, the S-isomer of omeprazole. Alimentary Pharmacology and Therapeutics, 15(10): 1563–1569. doi:10.1046/j.1365-2036.2001.01087.x.
Andrews, F.M., Sifferman, R.L., Bernard, W., Hughes, F.E., Holste, J.E., Daurio, C.P., Alva, R. & Cox, J.L. (1999). Efficacy of omeprazole paste in the treatment and prevention of gastric ulcers in horses. Equine Veterinary Journal. Supplement, 31(S29): 81-86.
Barton, A., Merle, R. & Gehlen, H. (2021). Efficacy of two oral omeprazole formulations for the therapy of equine gastric ulcer disease. In: Proceedings of the European College of Equine Internal Medicine Congress.
Begg, L.M. & O’Sullivan, C.B. (2003). The prevalence and distribution of gastric ulceration in 345 racehorses. Australian Veterinary Journal, 81(4): 199–201. doi:10.1111/j.1751-0813.2003.tb11469.x.
Bell, R., Kingston, J., Mogg, T. & Perkins, N. (2007). The prevalence of gastric ulceration in racehorses in New Zealand. New Zealand Veterinary Journal, 55(1):13–18. doi:10.1080/00480169.2007.36729.
Cargile, J.L., Burrow, J.A., Kim, I., Cohen, N.D. & Merritt, A.M. (2004). Effect of Dietary Corn Oil Supplementation on Equine Gastric Fluid Acid, Sodium, and Prostaglandin E2 Content before and during Pentagastrin Infusion. Journal of Veterinary Internal Medicine, 18(4): 545–549. doi:10.1111/j.1939-1676.2004.tb02583.x.
Doucet, M.Y., Vrins, A.A., Dionne, R., Alva, R. & Ericsson, G. (2003). Efficacy of a paste formulation of omeprazole for the treatment of naturally occurring gastric ulcers in training standardbred racehorses in Canada. Canadian Veterinary Journal, 44(7): 581 585.
Gough, S., Hallowell, G. & Rendle, D. (2020). A study investigating the treatment of equine squamous gastric disease with long‐acting injectable or oral omeprazole. Veterinary Medicine and Science, 6(2): 235–241. doi:10.1002/vms3.220.
Gough, S., Hallowell, G. & Rendle, D. (2022). Evaluation of the treatment of equine glandular gastric disease with either long-acting-injectable or oral omeprazole. Veterinary Medicine and Science, 8(2): 561-567. doi:10.1002/vms3.728.
Hepburn, R.J. & Proudman, C.J. (2014). Treatment of ulceration of the gastric glandular mucosa: Retrospective evaluation of omeprazole and sucralfate combination therapy in 204 sport and leisure horses (Abstract). In: Proceedings of the International Colic Symposium [Preprint]. doi:10.1111/jvim.12341/full.
Jansson, A., Harris, P., Davey, S.L., Luthersson, N., Ragnarsson, S. & Ringmark, S. (2021). Straw as an alternative to grass forage in horses — effects on post-prandial metabolic profile, energy Intake, behaviour and gastric ulceration. Animals, 11(8): 2197.
Lester, G., Robertson, I. & Secombe, C. (2008). Risk Factors for Gastric Ulceration in Thoroughbred Racehorses. Canberra: Australian Government: Rural Industries Research and Development Corporation.
Lester, G.D., Smith, R.L. & Robertson, I.D. (2005). Effects of treatment with omeprazole or ranitidine on gastric squamous ulceration in racing Thoroughbreds. Journal of the American Veterinary Medical Association, 227(10): 1636-1639.
Lind, T., Kyleback, A., Rydberg, L., Jonsson, A., Andersson, T., Hasselgren, G. & Rohss, K. (2000). Esomeprazole provides improved acid control vs omeprazole in patients with symptoms of GERD. Gastroenterology, 118(4): doi:10.1016/s0016-5085(00)82139-3.
Lorenzo-Figueras, M. & Merritt, A.M. (2002). Effects of exercise on gastric volume and pH in the proximal portion of the stomach of horses. American Journal of Veterinary Research, 63(11): 1481–1487. doi:10.2460/ajvr.2002.63.1481.
Luthersson, N., Nielsen, K.H., Harris, P. & Parkin, T.D.H. (2009). Risk factors associated with equine gastric ulceration syndrome (EGUS) in 201 horses in Denmark. Equine Veterinary Journal, 41(7): 625–630. doi:10.2746/042516409x441929.
Malmkvist, J., Poulsen, J.M., Luthersson, N., Palme, R., Christensen, J.W. & Søndergaard, E. (2012). Behaviour and stress responses in horses with gastric ulceration. Applied Animal Behaviour Science, 142(3–4): 160–167. doi:10.1016/j.applanim.2012.10.002.
Martineau, H., Thompson, H. & Taylor, D. (2009). Pathology of gastritis and gastric ulceration in the horse. Part 1: Range of lesions present in 21 mature individuals. Equine Veterinary Journal, 41(7): 638–644. doi:10.2746/042516409x464816.
Mason, L., Moroney, J. & Mason, R. (2019). Prophylactic therapy with omeprazole for prevention of equine gastric ulcer syndrome (EGUS) in horses in active training: A meta‐analysis. Equine Veterinary Journal, 51(1): 11–19. doi:10.1111/evj.12951.
Mönki, J., Hewetson, M. & Virtala, A. ‐M. K. (2016). Risk Factors for Equine Gastric Glandular Disease: A Case‐Control Study in a Finnish Referral Hospital Population. Journal of Veterinary Internal Medicine, 30(4): 1270–1275. doi:10.1111/jvim.14370.
Murray, M., Haven, M., Eichorn, E., Zhang, D., Eagleson, J. & Hickey, G. (1997). Effects of omeprazole on healing of naturally‐occurring gastric ulcers in Thoroughbred racehorses. Equine Veterinary Journal, 29(6): 425–429. doi:10.1111/j.2042-3306.1997.tb03153.x.
Murray, M., Schusser, G., Pipers, F. & Gross, S. (1996). Factors associated with gastric lesions in Thoroughbred racehorses. Equine Veterinary Journal, 28(5): 368–374. doi:10.1111/j.2042-3306.1996.tb03107.x.
Murray, M.J. & Eichorn, E.S. (1996). Effects of intermittent feed deprivation, intermittent feed deprivation with ranitidine administration, and stall confinement with ad libitum access to hay on gastric ulceration in horses. American Journal of Veterinary Research, 57(11): 1599–603.
Pedersen, S.K., Cribb, A.E., Windeyer, M.C., Read, E.K., French, D. & Banse, H.E. (2018). Risk factors for equine glandular and squamous gastric disease in show jumping Warmbloods. Equine Veterinary Journal, 50(6): 747–751. doi:10.1111/evj.12949.
Pickles, K.J., Black, K., Brunt, O. & Crane, M. (2020). Retrospective study of misoprostol treatment of equine glandular gastric disease. In: Proceedings of the European College of Equine Internal Medicine Congress. Online.
Rendle, D., Bowen, M., Brazil, T., Conwell, R., Hallowell, G., Hepburn, R., Hewetson, M. & Sykes, B. (2018). Recommendations for the management of equine glandular gastric disease. UK Vet: Equine, 2(Sup1): 2-11. doi:10.12968/ukve.2018.2.s1.3.
Rendle, D.I. (2017). Oral Esomeprazole As A Treatment For Equine Gastric Ulcer Syndrome Refractory To Oral Omeprazole. Equine Veterinary Journal, 49(S51): 25–26. doi:10.1111/evj.47_12732.
Rohss, K., Lundin, C., Rydholm, H. & Nyman, L. (2000). Esomeprazole 40 mg provides more effective acid control than omeprazole 40 mg. The American Journal of Gastroenterology, 95(9): 2432–2433. doi:10.1111/j.1572-0241.2000.02433.x.
Rohss, K., Wildersmith, C., Claarnilsson, C., Lundin, C. & Hasselgren, G. (2001). Esomeprazole 40 Mg provides more effective acid control than standard doses of all other proton pump inhibitors. Gastroenterology, 120(5): A419–A419. doi:10.1016/s0016-5085(01)82079-5.
Scheidegger, M.D., Gerber, V., Bruckmaier, R.M., Kolk, J.H. van der, Burger, D. & Ramseyer, A. (2017). Increased adrenocortical response to adrenocorticotropic hormone (ACTH) in sport horses with equine glandular gastric disease (GLANDULAR). The Veterinary Journal, 228: 7–12. doi:10.1016/j.tvjl.2017.09.002.
Sundra, T. (2021). Esomeprazole in the treatment of equine glandular gastric disease. UK-Vet Equine, 5(5): 216–219. doi:10.12968/ukve.2021.5.5.216.
Sykes, B., Sykes, K. & Hallowell, G. (2013). Efficacy of a Combination of a Unique Pectin‐Lecithin Complex (Apolectol®), Live Yeast and Magnesium Hydroxide in the Prevention of EGUS and Faecal Acidosis in Thoroughbred Racehorses: A Randomised, Blinded, Placebo Controlled Clinical Trial. Equine Veterinary Journal, 45(S44): 13. doi:10.1111/evj.12145_32.
Sykes, B.W. (2019). Courses for horses: Rethinking the use of proton pump inhibitors in the treatment of equine gastric ulcer syndrome. Equine Veterinary Education, 31(8): 441–446. doi:10.1111/eve.12894.
Sykes, B.W., Bowen, M., Habershon‐Butcher, J.L., Green, M. & Hallowell, G.D. (2019). Management factors and clinical implications of glandular and squamous gastric disease in horses. Journal of Veterinary Internal Medicine, 33(1): 233–240. doi:10.1111/jvim.15350.
Sykes, B.W., Hewetson, M., Hepburn, R.J., Luthersson, N. & Tamzali, Y. (2015a). European College of Equine Internal Medicine Consensus Statement--Equine Gastric Ulcer Syndrome in Adult Horses. Journal of Veterinary Internal Medicine / American College of Veterinary Internal Medicine, 29(5): 1288-1299. doi:10.1111/jvim.13578.
Sykes, B.W., Kathawala, K., Song, Y., Garg, S., Page, S.W., Underwood, C. & Mills, P.C. (2017a). Preliminary investigations into a novel, long-acting, injectable, intramuscular formulation of omeprazole in the horse. Equine Veterinary Journal, 49(6): 795-801. doi:10.1111/evj.12688.
Sykes, B.W., Sykes, K.M. & Hallowell, G.D. (2014). A comparison of two doses of omeprazole in the treatment of equine gastric ulcer syndrome: A blinded, randomised, clinical trial. Equine Veterinary Journal, 46(4): 416–421. doi:10.1111/evj.12191.
Sykes, B.W., Sykes, K.M. & Hallowell, G.D. (2015b). A comparison of three doses of omeprazole in the treatment of equine gastric ulcer syndrome: A blinded, randomised, dose–response clinical trial. Equine Veterinary Journal, 47(3): 285–290. doi:10.1111/evj.12287.
Sykes, B.W., Underwood, C., McGowan, C.M. & Mills, P.C. (2015c). The effect of feeding on the pharmacokinetic variables of two commercially available formulations of omeprazole. Journal of Veterinary Pharmacology and Therapeutics, 38(5): 500-503. doi:10.1111/jvp.12210.
Sykes, B.W., Underwood, C. & Mills, P.C. (2017b). The effects of dose and diet on the pharmacodynamics of esomeprazole in the horse. Equine Veterinary Journal, 49(5): 637–642. doi:10.1111/evj.12670.
Varley, G., Bowen, I.M., Habershon‐Butcher, J.L., Nicholls, V. & Hallowell, G.D. (2019). Misoprostol is superior to combined omeprazole‐sucralfate for the treatment of equine gastric glandular disease. Equine Veterinary Journal, 51(5): 575–580. doi:10.1111/evj.13087.
Vatistas, N., Snyder, J., Carlson, G., Johnson, B., Arthu, R., Thurmond, M., Zhou, H. & Lloyds, K.L.K. (1999). Cross‐sectional study of gastric ulcers of the squamous mucosa in Thoroughbred racehorses. Equine Veterinary Journal, 31(S29): 34–39. doi:10.1111/j.2042-3306.1999.tb05166.x.


