Dr. Stephanie Wood, PhD Equine Nutrition, PgDip, BSc (Hons), RNutr (Animal), R.Anim.Tech
Pituitary pars intermedia dysfunction (PPID) is a common disease predominantly diagnosed in horses and ponies, although it can also affect donkeys. It is a condition associated with ageing, with 15%-30% of horses and ponies over the age of 15 affected (Ireland & McGowan, 2018; EEG, 2021), although it has been diagnosed in equids as young as seven (Heinrichs et al., 1990). It is termed an endocrine disease, meaning that the organs producing hormones within the horse’s body are not functioning correctly. Specifically, the pars intermedia of the pituitary gland is affected, leading to the name pituitary pars intermedia dysfunction.
Pathophysiology of PPID
The pituitary gland sits at the base of the brain, suspended below the hypothalamus by the pituitary stalk (Figure 1). The pituitary gland comprises two distinct parts, the anterior lobe (also known as the adenohypophysis) located towards the front of the gland, and the posterior lobe (also known as the neurohypophysis) positioned towards the rear of the gland. Each lobe comprises of specific parts; the pars tuberalis, pars intermedia and pars distalis comprise the anterior lobe, and the pars nervosa and infundibular stalk comprise the posterior lobe (Dyce et al., 1996). The pituitary gland is known as a major endocrine gland as it produces hormones that directly influence other endocrine glands, and therefore the production of other hormones (Dyce et al., 1996). The production and secretion of hormones from the pituitary is regulated by the hypothalamus, or more accurately, by hormones secreted by neurosecretory cells of certain parts of the hypothalamus. Neurosecretory cells acting on the anterior lobe discharge their hormones into a capillary system serving the anterior lobe, whereas neurosecretory cells acting on the posterior lobe release their hormones directly into the posterior lobe (Frandson & Spurgeon, 1992; Dyce et al., 1996). Research has also shown that neurons secreting dopamine (dopaminergic neurons) originating from the periventricular nucleus of the hypothalamus, terminate in the pars intermedia (McFarlane et al., 2005). This difference is important to understand the pathophysiology of PPID.
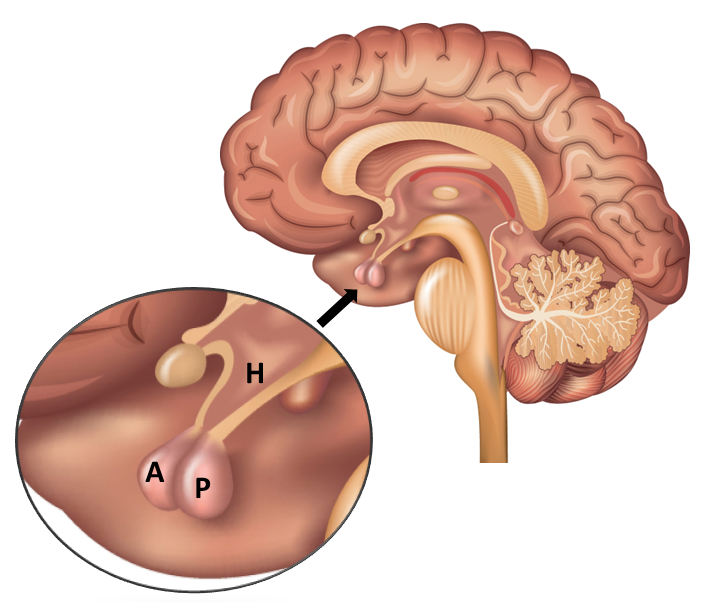
Figure 1. Location of the pituitary gland and hypothalamus in humans. Locations are similar in equids. A – Anterior lobe of pituitary gland, P – Posterior lobe of pituitary gland, H – Hypothalamus.
Both lobes of the pituitary gland secrete hormones that are vital for maintenance of body systems and health. When hormone secretion is below or above requirements imbalances develop, which over time lead to illness. In equids with PPID this imbalance relates to hormones produced by the pars intermedia of the anterior lobe of the pituitary gland. The change in hormone secretion is due to degeneration of the dopaminergic neurons (EEG, 2021), which occurs as part of the normal ageing process but may also be due to oxidative stress (Reed et al., 2018), with this degenerative loss being accelerated in some horses (Frank, 2015). This leads to PPID also being thought of as a neurodegenerative disease.
In non-PPID equids, dopaminergic neurons release dopamine that interacts with receptors on melanotrope cells which comprise the pars intermedia. Melanotrophs produce pro-opiomelanocortin (POMC) which is a prohormone that is enzymatically converted to the hormones alpha-melanocyte-stimulating hormone (α-MSH), corticotropin-like intermediate peptide (CLIP) and β-endorphin (Frank, 2015; Reed et al., 2018). Only a very small amount of adrenocorticotropic hormone (ACTH) is produced by the normally functioning pars intermedia. The production of POMC and its associated hormones is under the control of dopamine, with dopamine secretion inhibiting melanotrope POMC production in normal conditions, however in PPID affected equids, the degeneration of dopaminergic neurons reduces this inhibitory control. As such, melanotrophs become more active leading to an increase in the number of melanotrope cells (hyperplasia), enlargement of the pars intermedia and development of adenoma (non-cancerous tumour) within the pars intermedia (Frank, 2015; EEG, 2021). The overactive melanotrophs produce more POMC leading initially to increased α-MSH and CLIP secretion, followed by increased ACTH production as the disease progresses (Frank, 2015). It is the excessive secretion of these hormones, particularly ACTH, that is thought to lead to the clinical signs of PPID, although the exact processes involved are not fully understood.
In non-PPID animals ACTH is primarily secreted from the pars distalis of the anterior lobe in response to stimuli by corticotropin-releasing hormone (CRH) from the hypothalamus. Once secreted, ACTH acts on the adrenal cortex of the adrenal glands, stimulating the secretion of glucocorticoids (cortisol, corticosterone and cortisone) (Gallo-Payet et al., 2017). Cortisol has many functions although its main functions relate to the body’s response to physical and emotional stress, during which it stimulates an increase in available energy causing hyperglycaemia (raised blood glucose) and decreases sensitivity (Hart et al., 2016; Schemthaner-Reiter et al., 2021). In healthy equids cortisol levels are maintained within a normal range by a feedback system that in response to raised plasma cortisol levels, reduces CRH secretion from the hypothalamus leading to a consequential reduction in ACTH secretion. If ACTH levels are persistently raised the adrenal cortex becomes hyperplasic and circulating cortisol levels remain high. Increased cortisol levels have negative effects on the body, causing persistent high blood glucose, decreased insulin sensitivity and a reduced immune response that delays healing (Fortin et al., 2021). Such physiology explains many of the symptoms displayed by PPID affected equids, however recent research shows that only about 20% of PPID cases will present with enlarged adrenal cortex and increased circulating cortisol despite having increased ACTH levels (Reed et al., 2018). This suggests that other factors are important for the development of PPID symptoms.
Symptoms of PPID
The symptoms commonly exhibited by equids with PPID include being prone to laminitis, insulin dysregulation (ID), excessive hair growth, poor coat quality, failure to shed a thick winter coat, excessive sweating (hyperhidrosis), weight loss, muscle wastage, development of regional fat pads, excessive urine production and urination (polyuria), excessive thirst (polydipsia), recurrent infections, increased susceptibility to parasite infections, reduced response to pain, lethargy, change in appetite and even blindness (Reed et al., 2018; EEG, 2021). Of these symptoms, excessive hair growth is the most common (55%-80% of cases) and the increased risk of laminitis potentially the most debilitating.
Increased risk of laminitis
Why equids with PPID often suffer from chronic laminitis has been a key research focus over recent years. We know that ID is a key component of endocrinopathic laminitis (see Equine Metabolic Syndrome and Update on laminitis) and that ID occurs in 30%-60% of PPID cases, although prevalence could be as high as 77% (Horn et al., 2019; Tadros et al., 2019). Karikoski et al. (2016) confirmed the role of hyperinsulinemia in laminitis in a study comparing circulating insulin levels in a group of PPID horses. Hyperinsulinemia was present in all horses with laminitis and absent in those without laminitis. Such results indicate that laminitis may be a result of ID rather than PPID, whilst also explaining why PPID horses with hyperinsulinemia appear to be at the highest risk of developing laminitis. Research by Tadros et al. (2019) further support the role of high circulating insulin in laminitis, finding that plasma insulin concentrations increased with laminitis severity. How PPID and hyperinsulinemia are linked however is less clear. The role of cortisol continues to be considered, and recent research showing that equids with PPID and ID have higher circulating unbound cortisol (free cortisol fraction, FCF) compared to healthy equids whilst having similar total cortisol concentrations may explain this link (Hart et al., 2016; Vaughn et al., 2022). Free cortisol is biologically active and therefore promotes raised blood glucose and antagonises (inhibits) the effects of insulin, leading to the hyperinsulinemia often seen in equids with PPID. Research continues to investigate this link and determine other contributing factors to the high risk of laminitis that PPID equids demonstrate.
Management and feeding of PPID
Equids displaying clinical symptoms of PPID, or older than 10 years of age with chronic laminitis issues, should be assessed by a vet for the development of PPID, whilst testing for ID is recommended in all PPID cases (Durham et al., 2014; EEG, 2021). Upon PPID diagnosis the vet will lead the treatment and management plan that will incorporate drug therapy and husbandry recommendations. Pergolide remains the medication of choice, with regular blood tests used to monitor ACTH levels and response to treatment (EEG, 2021). Husbandry requirements will be dictated by issues associated with PPID. Correct hoof care to maintain hoof balance and increased monitoring of parasite burdens are some examples of the additional needs that PPID equids may have. Other considerations include regular dental care, monitoring of skin health due to increased susceptibility to skin infections and ensuring access to clean water is available at all times. Management of laminitis episodes should follow recommendations by your vet, aligning to those by Professor Menzies-Gow in Update on laminitis and should include providing appropriate pain relief and foot support alongside feeding an appropriate diet.
Feeding recommendations
Feeding recommendations for equids with PPID are influenced by the presence or absence of hyperinsulinemia and other associated health problems, and align to those for equids with EMS and ID. Like all equids fibre should be the main constituent of the diet for equids with PPID, with quality protein, vitamins, and minerals, provided to balance the diet. If additional energy is needed to maintain body weight options include higher energy fibre feeds such as alfalfa and oil-coated forages, high fat feeds such as linseed or oils, or appropriate concentrate feeds. For PPID equids all feeds should be low in starch and sugars to help maintain low blood glucose and insulin levels. Ideally feedstuffs should have a non-structural carbohydrate (NSC) level less than 10% of dry matter (DM) (Borgia et al., 2011; Harris et al., 2017; EEG, 2020) (Figure 2), which can preclude concentrate feeds, however there are now feeds and supplements on the market specifically designed to provide energy whilst having a low NSC content.
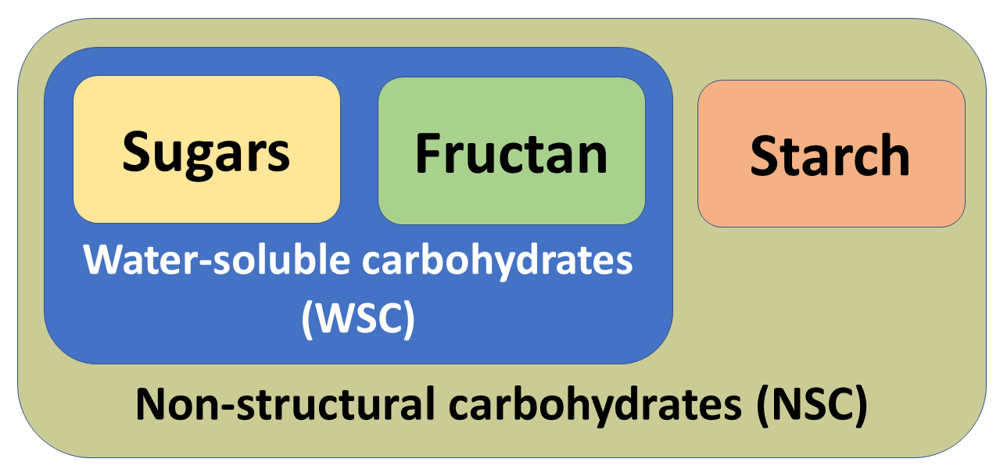
Figure 2. Composition of water-soluble carbohydrates (WSC) and non-structural carbohydrates (NSC) used to describe the nutritional properties of plants.
Forage should ideally be tested for its NSC content as there is no way of knowing the nutritional content without testing. Assumptions that meadow hays are lower in NSC are misplaced as the level of NSC accumulation in the growing plant, and therefore the preserved forage, varies with climate, stage of growth, health of the plant when harvested and geographical area (Richards et al., 2021). Hay is recommended over haylage for equids with metabolic issues due to its lower digestible energy (DE) content, which is an important consideration for overweight equids, although DE levels would also need confirming through laboratory analysis. Haylage preserved anaerobically and with a DM content of 50%-70% should have lower sugar levels than hay due to fermentation processes (see Forage options for horses) however many haylages have a higher DM content, limiting the degree of fermentation and leading to the production of wrapped hay rather than haylage. In these instances, the sugar levels may be higher than hay, particularly if the plant was cut at an earlier stage of growth.
Soaking continues to be used as a method to reduce sugar levels in hays, and current recommendations for PPID, ID and EMS equids are to soak hay for a minimum of 60 minutes (EEG, 2020) although longer times may be needed (Figure 3). The efficacy of soaking hay to achieve a NSC level below 10% will depend on the level of NSC prior to soaking and on the soaking conditions. Argo et al. (2015) report water-soluble carbohydrate (WSC – sugars and fructan) losses from grass hay with a starting WSC level of 18% DM. After 7 hours soaking at ambient temperature WSC levels decreased by 24% to 14% DM, whilst after 16 hours soaking (overnight) in the same conditions levels decreased by 41% to 10.5% DM. In warmer conditions the time to achieve WSC losses is reduced and therefore soaking time can be shortened (Longland et al., 2014) as demonstrated by Bochnia et al. (2021) who found that soaking for just 15 and 30 minutes at 22oC can reduce WSC content by 13%-24% and 23%-34%, respectively. In the same study it was also found that soaking hays of low WSC content (2.5%-6.6% WSC) had little effect on post-soaking WSC levels. Due to concerns about hygienic quality of long soaking times (Moore-Colyer et al., 2014), soaking duration should be shortened to 1-2 hours in warm weather (Durham et al., 2019). It is also highlighted that soaking causes loss of minerals and trace elements.

Figure 3. Soaking can be a useful way of reducing water-soluble carbohydrate (WSC) levels in hay although efficacy will be influenced by water temperature and soaking time.
The amount to feed will depend on the animal’s body condition. Those of lean condition should be fed 2%-2.5% of their body weight as suitable forages and high fat/oil feedstuffs. Animals that are overweight should be fed a diet and ration that promotes weight loss. For some animals replacing a portion of the hay ration with hygienic straw maybe enough to induce weight loss whilst allowing them to eat for extended periods of time and perform normal feeding behaviours. If straw is added to the diet it should be gradual to allow adaptation to the higher intake of indigestible fibre. Daily food intake may need to be limited to 1.5% body weight as DM per day (e.g. 6.75kg per day for a 450kg horse) to induce weight loss, although this should be guided by your vet or a qualified nutritionist (Shepherd et al., 2021). Feeding less than this amount may be required for equids resistant to weight loss however this is not recommended unless advised by your vet who should regularly monitor the animal’s response to such restricted food intake. When on restricted intake the use of slow feeders should also be considered as a way of prolonging eating time and supporting digestive health and allowing natural feeding behaviour.
Pasture access and intake
Similar to management and feeding recommendations, access to pasture will be guided by the presence of hyperinsulinemia and episodes or risk of laminitis, rather than a diagnosis of PPID per se. Non-structural carbohydrate levels of pasture vary considerably therefore management of intake is usually required for equids with PPID and ID who can consume large amounts if given free access. Methods of pasture restriction include limiting grazing area, limiting grazing time and use of a grazing muzzle. Limiting grazing area can be achieved using electric fencing and works well combined with strip grazing. Limiting grazing time is only effective if the amount of pasture available per animal is also limited, as equids are able to increase their intake rate to compensate for the shorter grazing period (Ince et al., 2011; Wood et al., 2012; Glunk et al., 2013). Limiting grazing time is most effective when the available pasture is sparse, preventing this compensatory behaviour. Use of grazing muzzles can considerably reduce pasture intake whilst allowing animals to move freely, exercise and socialise, however they should be introduced gradually to give time to become accustomed to wearing the muzzle and learning how to drink. If muzzles are worn for part of the day, once the muzzle is removed the same compensatory intake of pasture may be seen (Longland et al., 2016; Davis et al., 2020). For more in-depth information on restricting pasture intake see Managing our horse’s grass intake. During laminitic episodes animals should be removed from the pasture completely until the condition is under control and hoof structures are stable. Controlled pasture access may then be possible although some equids with ID are very sensitive to pasture and are more stable on dry-lot turnout areas and controlled feeding of forages.
Monitoring the amount of pasture available in a field can be challenging as fresh grass is often grazed as soon as it grows. As such, visual assessment of how much pasture there is available to eat is difficult. Sectioning off a small area of the field (1-2m2) with electric fencing prevents new growth being consumed and allows pasture availability to be monitored. The fenced area should be in parts of the field which are actively grazed and it should be moved every 1-2 weeks to ensure an accurate representation of the pasture being consumed.
Summary
Pituitary pars intermedia dysfunction is a disease affecting primarily older equids but which should be considered in equids over the age of 10 with recurrent laminitis. Abnormal hair growth and shedding, changes in body condition and composition, polyuria, polydipsia, and reduced immunity are the most common symptoms. Insulin dysregulation is present in some but not all PPID cases, but for those with hyperinsulinemia management and feeding should align to recommendations for ID and EMS equids. A diet low in starch and sugar, high in fibre and balanced for protein, vitamins, and minerals, is advocated alongside controlled pasture intake. Quality of life for equids with PPID is increased where feeding and management recommendations are combined with appropriate veterinary treatment.
References
Argo, C.McG, Dugdale, A.H.A., & McGowan, C.M. (2015). Considerations for the use of restricted, soaked grass hay diets to promote weight loss in the management of equine metabolic syndrome and obesity. The Veterinary Journal, 206: 170-177.
Bochnia, M., Pietsch, C., Wensch-Dorendorf, M., Greef, M., & Zeyner, A. (2021). Effect of Hay Soaking Duration on Metabolizable Energy, Total and Prececal Digestible Crude Protein and Amino Acids, Non-Starch Carbohydrates, Macronutrients and Trace Elements. Journal of Equine Veterinary Science, 101: 103452.
Borgia, L., Valberg, S., McCue, M., Watts, K., & Pagan, J. (2011). Glycaemic and insulinaemic responses to feeding hay with different non-structural carbohydrate content in control and polysaccharide storage myopathy-affected horses. Journal of Animal Physiology and Animal Nutrition, 95(6): 798-807.
Davis, K.M., Iwaniuk, M.E., Dennis, R.L., Harris, P.A., & Burk, A.O. (2020). Effects of grazing muzzles on behavior and physiological stress of individually housed grazing miniature horses. Applied Animal Behaviour Science, 231: 105067.
Durham, A,E., Frank, N., McGowan, C.M., Menzies-Gow, N.J., Roelfsema, E., Vervuert, I., Feige, K. & Fey, K. (2019) ECEIM Consensus Statement on Equine Metabolic Syndrome. Journal of Veterinary Internal Medicine, 33: 335-349
Durham, A.E., McGowan, C.M., Fey, K., Tamzali, Y., & van der Kolk, J.H. (2014). Pituitary pars intermedia dysfunction: diagnosis and treatment. Equine Veterinary Education, 26(4): 216-223.
Dyce, K.M., Sack, W.O., & Wensing, C.J.G. (1996). Textbook of Veterinary Anatomy, 2nd edn. New York, USA: W.B. Saunders Company.
Equine Endocrinology Group (2020). Recommendations for the Diagnosis and Treatment of Equine Metabolic Syndrome (EMS).
Equine Endocrinology Group (2021). Recommendations for the Diagnosis and Treatment of Pituitary Pars Intermedia Dysfunction (PPID). Available at: THIS LINK IS NO LONGER AVAILABLE (https://idppid.com/sites/idppid/files/pdf_download/2021 EEG PPID Recommendations (1).pdf)
Fortin, J.S., Hetak, A.A., Duggan, K.E., Burglass, C.M., Penticoff, H.B., & Schott II, H.C. (2021). Equine pituitary pars intermedia dysfunction: a spontaneous model of synucleinopathy. Scientific Reports, 11: 16036.
Frandson, R.D., & Spurgeon, T.L. (1992). Anatomy and Physiology of Farm Animals, 5th edn. Philadelphia, USA: Lippincott Williams & Wilkins.
Frank, N. (2015). Chapter 136 Pituitary Pars Intermedia Dysfunction. In: Sprayberry, K.A., & Robinson, N.E (eds.). Robinson's Current Therapy in Equine Medicine (7th edn) (pp 574-577). Missouri, USA: Saunders.
Gallo-Payet, N., Martinez, A., & Lacroix, A. (2017). Editorial: ACTH Action in the Adrenal Cortex: From Molecular Biology to Pathophysiology. Frontiers in Endocrinology, 8: 101.
Glunk, E.C., Pratt-Phillips, S.E., & Siciliano, P.D. (2013). Effect of Restricted Pasture Access on Pasture Dry Matter Intake Rate, Dietary Energy Intake, and Fecal pH in Horses. Journal of Equine Veterinary Science, 39: 421-426.
Harris, P.A., Ellis, A.D., Fradinho, M.J., Jansson, A., Julliand, V., Luthersson, N., Santos, A.S., & Vervuert, I. (2017). Review: Feeding conserved forage to horses: Recent advances and recommendations. Animal, 11(6): 958-967.
Hart, K.A., Wochele, D.M., Norton, N.A., McFarlane, D., Woolridge, A.A., & Frank, N. (2016). Effect of Age, Season, Body Condition, and Endocrine Status on Serum Free Cortisol Fraction and Insulin Concentration in Horses. Journal of Veterinary Internal Medicine, 30: 653-663.
Heinrichs, M., Baumgärtner, W., & Capen, C.C. (1990). Immunocytochemical Demonstration of Proopiomelanocortin-derived Peptides in Pituitary Adenomas of the Pars Intermedia in Horses. Veterinary Pathology, 27: 419-425.
Horn, R., Bamford, N.J., Afonso, T., Sutherland, M., Buckerfield, J., Tan, R.H.H., Secombe, C.J., Stewart, A.J., & Bertin, F.R. (2019). Factors associated with survival, laminitis and insulin dysregulation in horses diagnosed with equine pituitary pars intermedia dysfunction. Equine Veterinary Journal, 51: 440-445.
Ince, J., Longland, A.C., Newbold, J.C., & Harris, P.A. (2011). Changes in proportions of dry matter intakes by ponies with access to pasture and haylage for 3 and 20 hours per day respectively, for six weeks. Journal of Equine Veterinary Science, 31: 283.
Ireland, J.L., & McGowan, C.M. (2018). Epidemiology of pituitary pars intermedia dysfunction: A systematic literature review of clinical presentation, disease prevalence and risk factors. The Veterinary Journal, 235: 22-33.
Karikoski, N.P., Patterson-Kane, J.C., Singer, E.R., McFarlane, D., & McGowan, C.M. (2016). Lamellar pathology in horses with pituitary pars intermedia dysfunction. Equine Veterinary Journal, 48: 472-478.
Longland, A., Barfoot, C., & Harris, P. (2014). Effect of water temperature and agitation on loss of water-soluble carbohydrates and protein from grass hay: implications for equine feeding management. Vet Record, 174: 68
Longland, A.C., Barfoot, C., & Harris, P.A. (2016). Efficacy of Wearing Grazing Muzzles for 10 Hours per Day on Controlling Bodyweight in Pastured Ponies. Journal of Equine Veterinary Science, 45: 22-27.
McFarlane, D., Dybdal, N., Donaldson, M.Y., Millert, L., & Cribb, A.E. (2005). Nitration and Increased α-Synuclein Expression Associated with Dopaminergic Neurodegenration In Equine Pituitary Pars Intermedia Dysfunction. Journal of Neuroendocrinology, 17: 73-80.
Moore-Colyer, M.J., Lumbis, K., Longland, A., & Harris, P. (2014). The effect of five different wetting treatments on the nutrient content and microbial concentration in hay for horses. PLoS One, 9(11): e114079.
Reed, S.M., Bayly, W.M., & Sellon, D.C. (2018). Chapter 16 Disorders of the Endocrine System. In: Reed, S.M., Bayly, W.M., & Sellon, D.C (eds). Equine Internal Medicine (4th edn) (pp1029-1138). Missouri, USA: Saunders.
Richards, N., Nielsen, B., & Finno, C.J. (2021). Nutritional and Non-nutritional Aspects of Forage. Veterinary Clinics of North America: Equine Practice, 37(1): 43-61.
Schemthaner-reiter, M., Wolf, P., Vila, G., Luger, A. (2021). The Interaction of Insulin and Pituitary Hormone Syndromes. Frontiers in Endocrinology, 12: 626427.
Shepherd, M., Harris, P., & Martinson, K.L. (2021). Nutritional Considerations When Dealing with an Obese Adult Equine. Veterinary Clinics of North America: Equine Practice, 37(1): 111-137.
Tadros, E.M., Fowlie, J.G., Refsal, K.R., Marteniuk, J., & Schott II, H.C. (2019). Association between hyperinsulinaemia and laminitis severity at the time of pituitary pars intermedia dysfunction diagnosis. Equine Veterinary Journal, 51: 52-56.
Vaughn, S.A., Norton, N.A., & Hart, K.A. (2022). Circulating Hypothalamic-Pituitary-Adrenal Axis Hormones and Insulin Concentrations in Horses and Ponies. Journal of Equine Veterinary Science, 111: 103810.
Wood, S.J., Smith, D.G., Morris, C.J., & Cuddeford, D. (2012). The effect of pasture restriction on dry matter intake of foraging donkeys in the United Kingdom. In: Saastamoinen M., Fradinho M.J., Santos A.S., Miraglia N. (eds) Forages and grazing in horse nutrition. Forages and grazing in horse nutrition, vol 132. Wageningen Academic Publishers, Wageningen.


