Rebecca Allan
Equids live in an environment shared with different species and are exposed to several external irritants from which skin conditions can arise. Insect bite hypersensitivity (IBH) is an allergic skin disease in horses, and is a relapsing condition caused by the Culicoides species of biting midges (Papadopoulos et al., 2010). The first case was reported in France in 1840 and by the published research of Henry and Borey (1937), it was named ‘dermatose estivale récidivante du cheval´, meaning ‘recurrent summer dermatosis of the horse´. Since then, this condition has become a worldwide issue and has developed different terminologies such as sweet itch, Queensland itch, summer eczema or seasonal dermatitis, names which vary depending on your geographical location (Pooley, 2005). Any equine breed can succumb to the condition, with the prevalence of this disease estimated at 3-10% across Europe (Birras et al., 2021). In mainland UK, the onset of sweet itch is prevalent between April and the beginning of December (Baker et al., 1978) however in European countries where a warmer climate is present this disease can take place at any time of year.
CULICOIDES MIDGE
The most common species inducing IBH is the Culicoides which belongs to the family Ceratopogonidae (Papadopoulos et al., 2010). They are small insects comprising of two wings with an elongated thorax and segmented abdomen which ranges between approximately 1.0-2.5mm in length. Interestingly, due to their small size they are also known as no-see-ums as they generally go unnoticed and are not easily seen (Mullen et al., 2019). Female mouthparts are well adapted for biting and piercing tissues. They also have a longitudinal groove through which saliva passes whilst they feed on vertebrate blood. Generally, the females require vertebrate blood for reproductivity purposes. Males on the other hand, differ slightly in morphology as their mouthparts are greatly reduced so generally do not carry out bloodsucking behaviour and therefore are not thought to cause IBH (Mullen et al., 2019). Nevertheless, it is the saliva passed through by their blood sucking feeding mechanism that induces IBH in horses.
It is also thought that black flies of the species Simulium Vittaum may cause IBH. This was determined through antigen stimulation testing through which affected horses reacted to extracts from Simulium Vittaum, leading to the view that they may share similar allergens as Culicoides, and therefore be a potential cause of IBH, although more studies need to confirm this (Baselgia et al., 2010).
INSECT BITE HYPERSENSITIVITY - PATHWAY
As is assumed, the saliva from the bite of Culicoides midge is an allergen, which means the saliva contains a protein which triggers an allergic/ hypersensitivity reaction in the host (in this case the horse) (Langner, et al., 2009). The onset of these reactions is caused by the equine’s immune system to aid recovery. Therefore, to understand the complexity of the disease it is important to understand the physiological responses taking place within the horse’s body.
Immune system hypersensitivity reactions can be broadly classified in two main groups: antibody-mediated reactions and T-lymphocyte mediated reactions (Swiderski, 2000). An antibody is a protein used by the body to identify and neutralise foreign objects (in this case allergens from the Culicoides saliva) (Swiderski, 2000), whilst a T-lymphocyte is a type of white blood cell that has a number of key roles in horse’s immune responses (effectively fighting the allergen).
Hypersensitivity reactions are categorised into different types, dependant on the immune responses triggered. Originally IBH was classified as a Type I hypersensitivity reaction however recent studies have also shown Type IV hypersensitivity reactions to be involved.
Type I hypersensitivity reactions, also known as immediate reactions, are antibody mediated and involve immunoglobulin E (IgE) antibodies. These antibodies act within 15 minutes of the allergen’s presence by binding to receptors on mast cells and basophils which release histamine, finally recruiting eosinophils (white blood cells) to the allergic site, and consequently producing an inflammatory response (Wagner et al., 2006, Fettelschoss-Gabriel et al., 2018).
From investigations via intradermal testing, it was also noted that horses suffering from IBH recruited T helper cells (Th2 cells) and eosinophils to the injection site. As Type IV hypersensitivity is characterised by T-lymphocyte mediated reactions, this study confirmed the presence of Type IV hypersensitivity reactions in horses suffering from IBH. However, Type IV reactions are divided into 4 subclasses, and due to the Th2 cells involved, it is subclass Type IVb reactions involved in IBH (McKelvie et al., 1999, Fettelschoss-Gabriel et al., 2018). Type IV reactions are also known as delayed reactions and can occur 24hr after allergen exposure. In IBH, Th2 cells act by producing cytokines such as interleukin-5 (IL-5). The IL-5 cytokine targets and attracts eosinophils to the allergic site which all together results in the production of inflammatory responses (Spencer & Weller, 2010, Fettelschoss-Gabriel et al., 2018). When activated, eosinophils also release effector molecules such as histamine which have the effect of enhancing allergic responses that can cause tissue damage (McKelvie et al., 1999, Fettelschoss-Gabriel et al., 2018). Thus, making eosinophils a key cell type in IBH allergic responses as they are present in both Type I and Type IV reactions.
As can be seen in Table 1, it is a combination of these immunological pathways caused by the saliva of the midge which start off the disease internally and leads to the external clinical signs presented by affected horses.
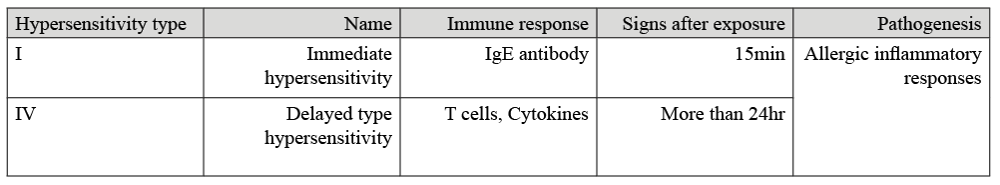
Table 1. Summary of hypersensitive reactions in IBH affected horses (Swiderski, 2000, Wagner et al., 2006)
CLINICAL SIGNS
As described in the immunological pathway of IBH, inflammation is the internal primary response leading to the condition. This initial inflammation to the allergic site consequently leads to irritation which results in the common clinical signs equines affected with this disease present (MacKay, 2000).
Baker et al. (1978) performed a study to determine the clinical aspects and histopathology of sweet itch. Fifteen affected horses were examined clinically by analysing skin scrapings. The age of the horses used in the study ranged from 18 months to 20 years old, meaning the results are applicable to a wide range of horses. Furthermore, the study took place from the end of April to the beginning of December, prime time for sweet itch to occur. Results showed clinical signs to be mainly pruritic (intense itching) with localised alopecia (hair loss), as well as evidence of skin abrasions. Additionally, horses who were commonly affected by the disease presented complete loss of hair over lesioned areas and rigid skin was noticed. The mane and top third of the tail were commonly affected and mostly de-haired (Figure 1). Björnsdóttir et al. (2006) supports these findings, reporting that the mane (93%) and the tail (72%) were the most affected areas in sweet itch horses, with the abdomen, head, side of the body, and chest also being affected, although to a lesser extent. It is to note, these clinical signs are a result of self-inflicted trauma as the horse tries to alleviate itself by itching. Additionally, secondary infections from other parasites such as mites may arise and produce further irritation, perpetuating lesion formation (Hastie, 2012).
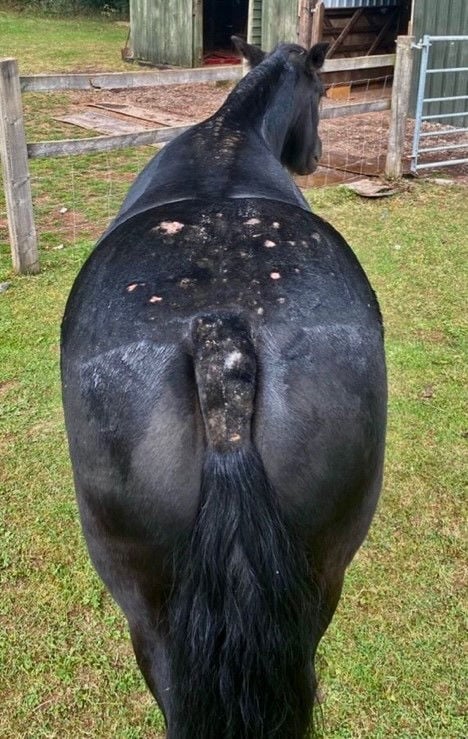
Figure 1. Common clinical signs presented by equines affected by IBH
DIAGNOSIS
The most common diagnosis for IBH is via visual assessment of the clinical signs presented by the horse. However, for further clarification and examination, intradermal tests are carried out by veterinary professionals.
Intradermal tests use extracts of environmental substances (in this case Culicoides extract) which are then injected into the dermis of the skin at specific volumes and concentrations (Figure 2). Injection sites are then assessed for a response, with the classic response being a wheal-and-flare response. The responses at the injection site are then measured at timed intervals such as at 30 minutes and at 4 hours post injection (Tahon et al., 2009, Lo Feudo et al., 2021). A positive result to the allergen would result in an inflammatory flare response. Thus, if your horse’s skin flares to the Culicoides spp. extract there is a strong likelihood of your horse having IBH.

Figure 2. Intradermal testing causing a wheal-and-flare response confirming IBH
MANAGEMENT AND PREVENTION
Management of IBH is based on reducing exposure to the biting midge. At present there is no definitive cure for sweet itch. Therefore, management and prevention methods are key to reduce affected horses’ exposure to the biting midges.
Culicoides midges are found worldwide, however they do have environmental and climate conditions which they favour. While biting midges have wings, they are poor flyers hence windy conditions do not favour their activity. As a general guideline, windless conditions with temperatures above 10℃ are ideal for their activity (Barbet, 2014). They also favour moist and humid surfaces such as moist mud, as this is where the females lay their eggs (Mullen et al., 2019). In practical terms, areas near water buckets, water throughs, streams and ponds which have moist terrain nearby, are the areas where midges are going to thrive, although midges can travel up to 2km from their breeding ground, meaning their source may not be obvious, and therefore more challenging to avoid (Mullen et al., 2019).
By knowing the midge’s activity, a management and prevention plan can be thought out. It is also of high importance to be proactive and start these measures before April to prevent the onset of sweet itch.
- As mentioned, midges thrive on moist and damp conditions, such as near water throughs in fields or on droppings. Therefore, removing faeces from fields should be regular practice and avoiding dampness near water buckets by not letting them overfill is advised. In addition, avoiding turning out near water sources such as ponds and streams is preferable if facilities allow.
- Midges are most active during dusk and dawn, therefore preventing your horse from being turned out in the field during these times of day can help to limit exposure.
- Due to females laying their eggs on damp surfaces and midges generally being poor flyers – exposed well drained fields for your horse’s turnout are the best option.
As well as daily yard management, sweet itch rugs can also help reduce horses’ exposure to the allergen. Sweet itch rugs are designed with a fine mesh to prevent the midge coming into direct contact with the horse, effectively acting as a barrier (Figure 3). Sweet itch rugs also provide belly coverage which standard fly rugs do not offer. Although costly, sweet itch rugs can be of great help to manage the disease.

Figure 3. Sweet itch rugs as a control method against midge bites
Furthermore, Baker et al. (2015) performed a study to determine whether the addition of insecticide-treated netting (ITN) in stables could provide protection for horses from Culicoides spp. in the UK. It was concluded that ITN of 1.6mm in aperture does have the potential to offer protection from Culicoides midges and reduce the biting of these insects. However, this study failed to conclude on the most effective insecticides available in the UK as results varied. Thus, this study provides evidence that fine netting in stables can help deter the biting midges.
NUTRITIONAL SUPPORT
As well as daily routine management and physical barriers such as rugs and stable nettings, soothing the itching to keep your horse as comfortable as possible is also beneficial. This can be achieved through nutritional supplementation providing support from the inside out to support skin health.
Dietary supplementation with essential fatty acids (EFAs) may be useful in equine allergic conditions. Essential fatty acids are known to be crucial in maintaining skin barrier function and have a recognised role in inflammatory processes. Furthermore, Omega-3 fatty acids are especially renowned for their anti-inflammatory properties (Hess & Ross-Jones, 2014). Supplements high in Omega-3 fatty acids are those such as linseed oil, algae oil, and fish oils. Linseed oil is high in alpha-linolenic acid which theoretically is converted to eicosapentaenoic acid (EPA) and possibly into docosahexaenoic acid (DHA) (Harper et al., 2006). Thus, making it a suitable aid to support healthy skin tissue. However, according to Weldon et al. (2007), DHA is more effective in reducing inflammation than EPA. For this reason, algae oil is especially beneficial as it contains high levels of DHA exhibiting potent anti-inflammatory actions (Nauroth, et al., 2010). Interestingly, a study by Elzinga et al. (2019) found DHA-rich microalgae supplementation to have potential immune-modulating and anti-inflammatory properties for horses with Equine Metabolic Syndrome (EMS). Therefore, algae oil supplementation may help soothe and alleviate irritated skin as well as support the immune system.
Vitamin E is recognised as a powerful antioxidant, with human research indicating that it is regularly secreted on the facial skin surface, as this area is most exposed to environmental damage, and that it may play a pivotal role in the removal of free radicals from the skin, reducing cell damage and potentially benefiting skin health (Thiele et al., 1999, Williams & Carlucci, 2006). A study conducted by Plevnik et al. (2014) on dogs suffering from atopic dermatitis, showed improved skin lesions in dogs supplemented with Vitamin E compared to those on the placebo treatment. Improvement in the clinical signs such as excoriation (skin biting, chewing or licking) and alopecia were noted after 8-weeks Vitamin E supplementation, hence the view that Vitamin E may have beneficial effects on the skin's condition.
Vitamin E is also recognised for its immune-supporting actions (Bendich, 1993). A study conducted by Petersson et al. (2010) found that Vitamin E supplementation of predominantly older horses enhanced their general cell-mediated immunity (immune response driven by T cells, cytokines, and macrophages) and humoral immune functions (immune response mediated by antibodies). Vitamin E supplementation may therefore be particularly beneficial for horse’s suffering from sweet itch to aid their overall immune system.
Herbal nutrition ptions to support skin health from the inside out are also available. Table 2 offers some herbal options for optimum skin condition and to help alleviate itchiness, although herbal therapies are not limited to the herbs listed as there are many other herbs such as Nettle, Clivers, and Chamomile, that can help soothe sensitive or irritated areas.
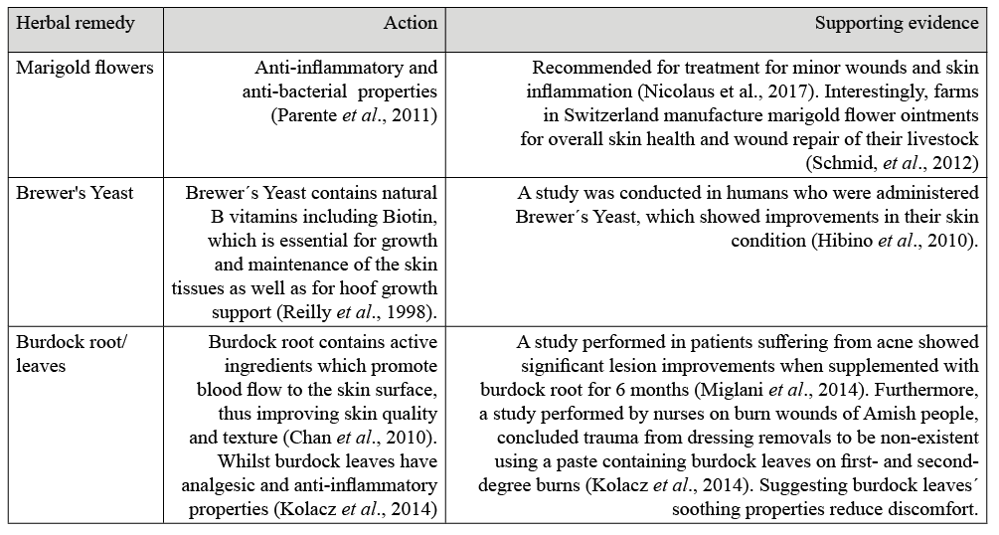
Table 2. Herbal Remedies with their function and scientific evidence
FUTURE TREATMENT
Although currently a specific treatment has not been established to deal with IBH, sweet itch has become a key research area, with studies showing that a vaccine may effectively treat the condition.
As previously described, IBH involves Type I and Type IV hypersensitivity reactions. The Type IV hypersensitivity reactions are characterised by Th2 cells which produce IL-5 cytokines attracting eosinophils, creating an inflammatory response. Eosinophils account for a large portion of the symptoms presented by IBH. Due to these series of reactions, Fettelschoss-Gabriel et al. (2018) decided to target the IL-5 cytokine as it is a master regulator for eosinophils as it is in charge of eosinophils´ survival, development, and activation. It was thought that blocking IL-5 cytokine would be a safe and efficient way in reducing eosinophil-mediated inflammation. Previous studies using mice treated with IL-5 antibodies resulted in reduced blood eosinophils, meaning eosinophil counts could be regulated by limiting IL-5 stimulation (Zou et al., 2010). Also, studies in human patients suffering from eosinophilic-mediated diseases such as eosinophil-mediated asthma and hyper-eosinophilia have also shown positive results. Patients were treated with antibodies targeting IL-5 cytokine´s action, significantly reducing eosinophil counts. Due to this treatment, the clinical signs presented by patients with inflammatory associated diseases were improved (Roufosse, 2018). Thus, these two studies led to the investigations into the vaccine for horses.
The vaccine was created on a novel, virus like particle (VLP) platform based on the cucumber mosaic virus. This contained the tetanus toxoid (inactivated toxin) universal T cell epitope (specific part of the antigen where the antibody binds itself to) also known as CMVTT. CMVTT enhances T helper cell responses for antigens displayed on the VLP surface. Thus, the vaccine consisted of equine IL-5 (eIL-5) chemically linked to CMVTT-VLPs which induced anti-eIL-5 antibodies in horses (Fettelschoss-Gabriel et al., 2018).
Icelandic horses suffering from IBH were used in the Fettelschoss-Gabriel et al. (2018) study to trial the vaccine. The results showed that the vaccine induced anti-IL-5 antibodies in horses and eosinophil-mediated symptoms were treated. There was no longer correlation between eosinophil counts and lesion scores as it was thought the absence of IL-5 due to vaccination meant eosinophils did not enter tissues. Alongside the internal response to the vaccine, clinical signs of sweet itch also improved when horses were vaccinated in comparison to the same horses the previous season and with those horses on the placebo-controlled experiment. The benefit of this vaccine is that when administered, the antibodies are self-made by your horse’s immune system meaning this vaccine can be administered before the IBH season starts, so antibody production increases during IBH season and decreases again during unaffected times of year.
Overall, eIL-5-CMVTT is the first anti-cytokine vaccine to have shown clinical efficacy for the treatment of equine IBH disease. Targeting IL-5 cytokine limited the development of eosinophils and the skins’ reaction, improving clinical signs.
SUMMARY
Insect bite hypersensitivity is a worldwide disease caused mainly by the saliva of female Culicoides species of the biting midge. The onset of this disease is based on the horse’s immune system exerting Type I and Type IV hypersensitivity reactions which then induce inflammation at the allergic site. This inflammation then triggers the presentation of external clinical signs, with the most common being intense itching and hair loss at the base of the mane and tail. Insect bite hypersensitivity can be determined through clinical signs but for further evidence intradermal tests can take place. Management procedures to minimise the exposure of the biting midge are key principles to control this disease as well as the physical barriers such as sweet itch rugs and stable netting. Likewise, nutritional supplementation also plays a crucial role in soothing irritation and improving skin health, and so in keeping horses comfortable. Furthermore, research has demonstrated that the vaccine (eIL-5-CMVTT) improves clinical signs of IBH in affected horses by targeting IL-5 cytokine, offering hope to the treatment and management of this incurable disease.
REFERENCES
Baker, K.P. & Quinn, P.J., (1978). A Report on Clinical Aspects and Histopathology of Sweet Itch. Equine Veterinary Journal, 10 (4): 243-248.
Baker, T., Carpenter, S., Gubbins, S., Newton, R., Lo lacono, G., Wood, J. & Harrup, L.E., (2015). Can insecticide-treated netting provide protection for Equids from Culicoides biting midges in the United Kingdom? Parasites & Vectors, 8: 604.
Barbet, J.L., (2014). Chapter 59 - Ectoparasites of Horses. In: Debra, C., Sellon, Maureen, T.L., (eds.). Equine Infectious Diseases, W.B. Saunder: UK
Baselgia, S., Doherr, M.G., Mellor, P., Torsteinsdottir, S., Jermann, T., Zurbriggen, A., Jungi, T. & Marti, E., (2010). Evaluation of an in vitro sulphidoleukotriene release test for diagnosis of insect bite hypersensitivity in horses. Equine Veterinary Journal, 38(1): 40-46.
Bendich, A., (1993). Physiological role of antioxidants in the immune system. Journal of Dairy Science, 76 (9): 2789-2794.
Birras, J., White, S.J., Jonsdottir, Sigridur., Novotny, E.N., Ziegler, A., Wilson, A.D., Frey, R., Torsteinsdottir, S., Alcocer, M. & Marti, E., (2021). First clinical expression of equine insect bit hypersensitivity is associated with co-sensitization to multiple Culicoides allergens. PLoS One, 16(11): e0257819.
Björnsdóttir, S., Sigvaldadóttir, J., Broström, H., Langvad, B. & Sigurösson, Á., (2006). Summer eczema in exported Icelandic Horses: influence of environmental and genetic factors. Acta Veterinaria Scandinavica, 48 (1): 3.
Chan, Y., Cheng, L., Wu, J., Chan, E., Kwan, Y., Lee, S.M., Leung, G.P., Yu, P.H. & Chan, S., (2010). A review of the pharmacological effects of Arctium Lappa (burdock). Inflammopharmacology, 19(5): 245-254.
Elzinga, S.E., Betancourt, A., Stewart, J.C., Altman, M.H., Barker, V.D., Muholland, M., Bailey, S., Brennan, M. & Adams., A.A., (2019). Effects of Docosahexaenoic Acid-Rich Microalgae Supplementation on Metabolic and Inflammatory Parameters in Horses with Equine Metabolic Syndrome. Journal of Equine Veterinary Science, (83): 102811
Fettelschoss-Gabriel, A., Fettelschoss, V., Thoms, F., Giese, C., Daniel, M., Olomski, F., Kamarachev, J., Birkmann, K., Bühler, M., Kummer, M., Zeltins, A., Marti, E., Kündig, T.M. & Bachmann, M.F., (2018). Treating insect-bite hypersensitivity in horses with active vaccination against IL-5. The Journal of Allergy and Clinical Immunology, 142(4): 1194-1205.e3
Harper, C., Edwards, M., DeFilipis, A. & Jacobson, T., (2006). Flaxseed Oil Increases the Plasma Concentrations of Cardioprotective (n-3) Fatty Acids in Humans. The Journal of Nutrition, 136(1): 83-87
Hastie, P.S. (2012). Skin diseases. In: Ivens, Philips (eds.). The BHS Veterinary Manual. 2nd Edition. Kenilworth Press: UK
Henry A. & Borey L. (1937). Dermatose estivale recidivante du cheval. Pathologie et therapeutique. Recueil de Medecine Veterinaire 113, 65-78.
Hess, T. & Ross-Jones, T., (2014). Omega-3 fatty acid supplementation in horses. Revista Brasileira de Zootecnia, 43(12).
Hibino, S., Hamada, U., Takahashi, H., Watanabe, M., Nozato, N. & Yonei, Y., (2010). Effects of Dried Brewer’s Yeast on Skin and QOL. Anti-Ageing Medicine, 7(4): 18-25.
Kolacz, N., Jaroch, M., Bear, M. & Hess, R., (2014). The Effect of Burns & Wounds (B&W)/Burdock Leaf Therapy on Burn-Injured Amish Patients. Journal of Holistic Nursing, 32(4): 327-340.
Langner, K.F., Jarvis, D.L., Nimtz, M., Heselhaus, J.E., McHolland, L.E., Leibold, W. & Drolet, B.S., (2009). Identification, expression, and characterisation of a major salivary allergen (Cul s 1) of the biting midge Culicoides sonorensis relevant for summer eczema in horses. International Journal for Parasitology, 39 (2).
Lo Feudo, C.M., Stucchi, L., Alberti, E., Conturba, B., Zucca, E. & Ferrucci, F. (2021). Intradermal testing results in horses affected by mild-moderate and severe equine asthma. Animals, 11(7): 2086.
MacKay, R. J., (2000). Inflammation in Horses. Veterinary Clinics of North America: Equine Practice, 16(1): 15-27.
McKelvie, J., Foster, A., Cunningham, F. & Hamblin, A., (1999). Characterisation of lymphocyte subpopulations in the skin and circulation of horses with sweet itch (Culicoides hypersensitivity). Equine Veterinary Journal, 31(6): 466-472.
Miglani, A. & Manchanda, R.K., (2014). Observational study of Arctium lappa in the treatment of acne vulgaris. Homeopathy, 103 (3): 203-207.
Mullen, Gary, R., & Murphree, C. S. (2019). Biting Midges (Ceratopogonidae). In: Durden, Lance, A.(eds.). Medical and Veterinary Entomology. 3rd ed. Academic Press: USA
Nauroth, J.M., Liu, Y.C., Elswyk, M.V., Bell, R., Hall, E.B., Chung, G., & Arterburn, L.M. (2010). Docosahexaenoic acid (DHA) and docosapentaenoic acid (DPAn-6) algal oils reduce inflammatory mediators in human peripheral mononuclear cells in vitro and paw edema in vivo. Lipids, 45(5): 375-384.
Nicolaus, C., Junghanns, S., Hartmann, A., Murillo, R., Ganzera, M. & Merfort, I. (2017). In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. Journal of Ethnopharmacology, 196: 94-103.
Papadopoulos, E., Rowlinson, M., Bartram, D., Carpenter, S., Mellor, P. & Wall, R. (2010). Treatment for horses with cypermethrin against the biting flies Culicoides nubeculosus, Aedes aegypti and Culex quinquefasciatus. Veterinary Parasitology, 169(1-2): 165-171.
Parente, L. M., Lino Júnior, R., Tresvenzol, L. M., Vinaud, M. C., de Paula, J. R. & Paulo, N. M., (2011). Wound Healing and Anti-Inflammatory Effect in Animal Models of Calendula officinalis L. Growing in Brazil. Evidence-based complementary and alternative medicine, 2012: 2012375671-375671.
Petersson, K.H., Burr, D.B., Gomez-Chiarri, M. & Pettersson- Wolfe, C.S., (2010). The influence of Vitamin E on immune function and response to vaccination in older horses. Journal of Animal Science, 88(9): 2950-2958.
Plevnik, A.K., Salobir, J, Levart, A., Kalcher, G. T., Nemec, A.S. & Kotnik, T. (2014). Vitamin E supplementation in canine atopic dermatitis: improvement of clinical signs and effects on oxidative stress markers. Veterinary Record, 175(22): 560.
Pooley, H. (2005). Conditions and Diseases. In: Beaton, J (eds.). Your horse´s skin. New Era Printing Company Ltd: China.
Reilly, J. D., Cottrell, D. F., Martin, R. J. & Cuddeford, D. J. (1998). Effect of supplementary dietary biotin on hoof growth and hoof growth rate in ponies: a controlled trial. Equine Veterinary Journal. (S.26): 51-57.
Roufosse, F., (2018). Targeting the Interleukin-5 Pathway for Treatment of Eosinophilic Conditions Other than Asthma. Frontiers in Medicine, 5: 49.
Schmid, K., Ivemeyer, S., Vogl, C., Klarer, F., Meier, B., Hamburger, M. & Walkenhorst, M., (2012). Traditional use of herbal remedies in Livestock by Farmers in 3 Swiss Cantons (Aargau, Zurich, Schaffhausen). Forschende Komplementärmedizin, 19(3): 125-136.
Spencer, L.A. & Weller, P.F., (2010). Eosinophils and Th2 immunity: contemporary insights. Immunology & Cell Biology, 88 (3): 250-256.
Swiderski, C. E., (2000). Hypersensitivity Disorders in Horses. Veterinary Clinics of North America: Equine Practice, 16(1): 131-151.
Tahon, L., Baselgia, S., Gerber, V., Doherr, M., Straub, R., Robinson, N. & Marti, E., (2009). In vitro allergy tests compared to intradermal testing in horses with recurrent airway obstruction. Veterinary Immunology and Immunopathology, 127(1-2): 85-93.
Thiele, J. J., Weber, S.U., & Packer, L., (1999). Sebaceous gland secretion is a major physiologic route of Vitamin E delivery to skin. Journal of Investigative Dermatology, 113(6): 1006 – 1010.
Wagner, B., Miller, W.H., Morgan, E.E., Hillegas, J.M., ERB, H.N., Leibold, W. & Antczak, D.F., (2006). IgE and IgG antibodies in skin allergy of the horse. Veterinary Research, 37(6): 813-825.
Weldon, S.M., Mullen, A.C., Loscher, C.E., Hurley, L.A. & Roche, H.M., (2007). Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. Journal of Nutritional Biochemistry, 18(4): 250-258.
Williams, C. & Carlucci, S., (2006). Oral vitamin E supplementation on oxidative stress, vitamin, and antioxidant status in intensely exercised horses. Equine Veterinary Journal, 38(S36): 617-621.
Zou, Y., Sonderegger, I., Lipowsky, G., Jennings, G.T., Schmitz, N., Landi, M., Kopf, M. & Bachmann, M.F., (2010). Combined vaccination against IL-5 and eotaxin blocks eosinophilia in mice. Vaccine, 28 (18): 3192-3200.


