Dr. Stephanie Wood, PhD Equine Nutrition, PgDip,
BSc (Hons), RNutr (Animal), R.Anim.Tech
Essential fatty acids have become a common topic when discussing equine nutrition, mainly due to our increased understanding of their integration into cells and tissues, and their important functions in physiological processes. To understand why these compounds are classed as essential in the diet we need to review their structure and function
WHAT ARE ESSENTIAL FATTY ACIDS?
We first need to understand lipids as a nutrient classification and the terminology used to describe them. Lipids is the term used to describe fats, waxes and oils, with each of these lipid types having their own properties, although all lipids are insoluble in water (McDonald et al., 2011). In plants, lipids form waxes that protect the plant’s structures, and oils which are mainly found in the seeds and nuts and that store energy. In animals, lipids are the main form of storing energy, mainly as adipose tissue (fat). Lipids also form animal cell membranes, hormones and are precursors for cells involved in immune responses (McDonald et al., 2011). Fat is the term used for describing lipids that are solid at room temperature (22-23℃) whereas oils are liquid at this temperature, although often these terms are used interchangeably to describe the lipid content of foods (Frankel, 2005).
FATTY ACIDS
Fatty acids are chains of carbon atoms linked to hydrogen atoms, with this chain attached to a carboxyl group at one end (Figure 1a). The carbon in the carboxyl group is designated carbon 1 and the final carbon at the terminal end (methyl end) as the omega carbon (McDonald et al., 2011). The number of carbons in the chain influences whether the fatty acid is referred to as a short chain (contains 1-6 carbons), medium chain (contains 7-12 carbons), or long chain (more than 12 carbons) fatty acid (Schönfeld & Wojtczak, 2016). Short chain fatty acids (SCFA) and medium chain fatty acids (MCFA) are abundant in nature although in smaller quantities than long chain fatty acids (LCFA), with LCFA containing 16 and 18 carbons forming the majority of fatty acids.
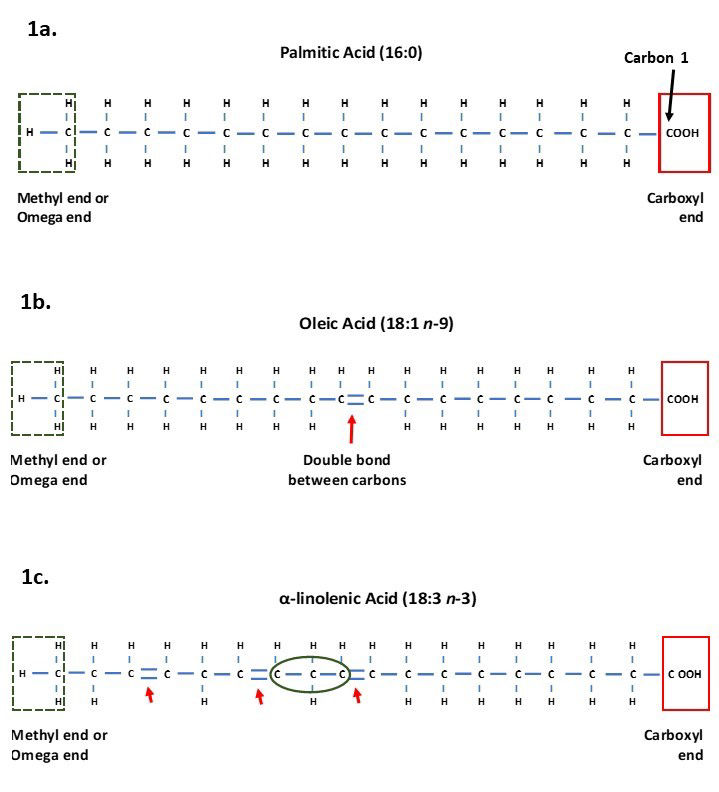
Figure 1. Structure of fatty acids showing the terminal methyl end, also known as the omega end in the green dashed box, and the carboxyl end and carbon 1 in the red solid box.
a) Palmitic acid which is a saturated long chain fatty acid containing 16 carbons and no double bonds (16:0).
b) Oleic acid containing 18 carbons and one double bond (highlighted by arrow) making it an unsaturated LCFA.
c) α-linolenic acid containing 18 carbons and three double bonds making it an unsaturated PUFA. The green circle shows a methylene group that is found between carbons with double bonds.
Fatty acids are also described according to the number of double bonds between carbons in their chain. Each carbon has four binding sites for binding to hydrogen atoms and their neighbouring carbons. When all the carbons in the chain are connected to each other via single bonds, and all the hydrogen sites are already bound, the fatty acids are said to be saturated as there are no spare binding sites (Horton et al., 1996; McDonald et al., 2011). Saturated fatty acids are straight in structure and so can pack tightly together, meaning they can form more solid fat than those containing unsaturated fatty acids (Horton et al., 1996).
Unsaturated fatty acids have one or more carbon atoms which have a double bond between them and their neighbouring carbon (Figure 1b). This means they still have space for additional hydrogen to join the chain, making them unsaturated with hydrogens (Horton et al., 1996; McDonald et al., 2011). Unsaturated carbons have bends in their chains at the site of the double bonds. These bends mean the fatty acids cannot lie as tightly together as saturated fatty acids, resulting in them often being in liquid form (Horton et al., 1996).
The number of double bonds leads to whether the fatty acid is referred to as a monounsaturated or polyunsaturated fatty acid (PUFA). Monounsaturated fatty acids have a single double bond whereas PUFA have two or more double bonds. Animal cells cannot add double bonds on the last nine carbons from the omega carbon (McDonald et al., 2011), therefore fatty acids requiring double bonds closer than nine carbons cannot be synthesised and so must be supplied in the diet. Such fatty acids are classed as essential fatty acids.
FATTY ACID NOMENCLATURE
Fatty acids are stated as the number of carbon atoms followed by the number of double bonds.

When fatty acids contain double bonds either the number of double bonds or the position of the double bonds is provided. In the nutrition field, the number of double bonds is stated along with the position of the first double bond from the omega carbon. For example, Oleic acid, the main fatty acid in olive oil, is a monounsaturated fatty acid stated as 18:1 n-9, meaning it contains 18 carbons and one double bond which is positioned at the ninth carbon from the omega carbon (Figure 1c). This nomenclature leads to the popular terms Omega-3, Omega-6 and Omega-9 fatty acids which are used in human and animal nutrition to group PUFA by the position of the first double bonds from the omega carbon. Omega-3 fatty acids are those with the first double bond on the third carbon from the omega carbon (n-3), Omega-6 fatty acids those with the first double bond on the sixth carbon (n-6), and Omega-9 fatty acids being those with the first double bond on the ninth carbon (n-9). For PUFA where there are multiple double bonds, the position of the other double bonds after that depicted by n- assumes that there is one carbon that is linked to two other carbons by single bonds (known as a methylene group) between double bonds. This means there are three carbon atoms before the next double bond occurs (Figure 1c).
ESSENTIAL FATTY ACIDS
As previously stated, animals (and humans) are unable to produce fatty acids with double bonds closer than nine carbons to the omega end. This means that long chain Omega-3 and Omega-6 fatty acids cannot be produced as the double bond occurs before the ninth carbon (McDonald et al., 2011), meaning a dietary source of these fatty acids should be fed. Nutritionally important fatty acids, their sources, number, and position of double bonds are shown in table 1. From table 1 it is evident that providing dietary sources of essential fatty acids is relatively straight forward as they are present in feeds commonly fed to equids. Horses consuming cereal grains will be consuming more of the Omega-6 fatty acids than they would on a forage or pasture-based diet. Of interest are the different proportions of fatty acids present in oils commonly fed to horses (Table 2). When feeding oil, the primary focus may be on the amount of calories that the oil can provide, meaning the fatty acid profile may be a secondary consideration, however the influence fatty acids can have on physiological processes mean the fatty acid profile of the oil fed may also need to be considered.
FUNCTIONS OF FATTY ACIDS
Individual, free fatty acids only occur in trace amounts in living cells and mainly occur joined (esterified) to a glycerol molecule to form glycerolipids (Frankel, 2005), including triglycerides and phospholipids. Triglycerides are formed by one glycerol molecule which has three carbons, joining with a fatty acid at each of these carbons (three fatty acids in total). Triglycerides are important storage lipids in animals and plants and are the main constituents of oils and fats, which contain mixtures of different triglycerides.
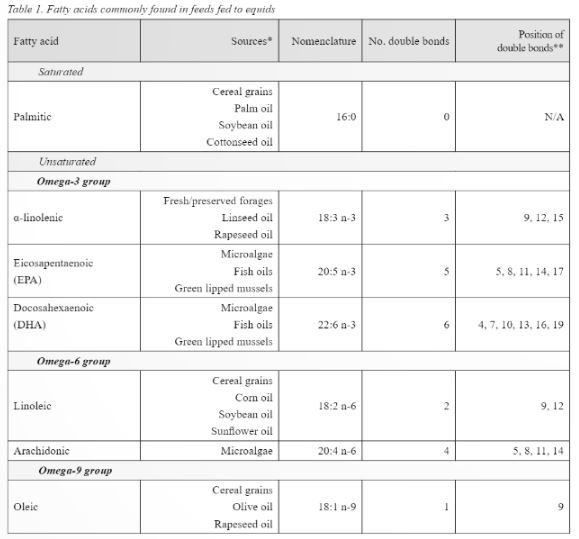
* Sourced from McDonald et al. (2011), Rosentrater & Evers (2018), Glasser et al. (2013), Maples (2013).
** Position of double bonds indicated by carbon number from the carboxyl end.
Triglycerides containing predominantly saturated fatty acids are found in solid fats due to their ability to pack tightly together, whereas triglycerides in oils predominantly contain unsaturated fatty acids, therefore oils are richer sources of essential Omega-3 and Omega-6 fatty acids than fats.
Phospholipids are important structural lipids that form cell membranes in animal and plant cells. Cell membranes are vital to life and health as they form the boundaries of cells and their compartments, allowing them to function as separate entities with specific purposes (Goodman, 2020). Phospholipids are formed by one glycerol molecule joining to two fatty acids and one phosphoric acid. The fatty acids are insoluble in water making them hydrophobic (water hating) and are termed non-polar, whereas the phosphoric acid is hydrophilic (water loving) and termed polar. The water loving and water hating properties of phospholipids enable them to form lipid bilayers which comprise cell membranes (Figure 2). The fatty acid within the bilayers influences the fluidity of the cell membrane, with phospholipids containing more unsaturated fatty acids having greater fluidity than those containing more saturated fatty acids (Horton et al., 1996; Goodman, 2020).
* Data adapted from Sargent (1997)
- Data not reported
INFLAMMATORY ROLE OF FATTY ACIDS
Essential fatty acids have received considerable attention due to their role in inflammatory processes. Inflammation is the body’s normal defence mechanism against injury and infection and is required for the destruction and removal of pathogens and repair of affected tissues (Calder, 2010, 2015). We all know the heat, swelling, redness, pain and reduced movement that signify inflammation, and these symptoms reflect the increased blood supply and infiltration of inflammatory mediators at the site of inflammation (James et al., 2000; Calder, 2010). These responses are important and help to protect the body however when inflammation continues over a prolonged period tissues can become damaged (Duvall & Levy, 2016). To avoid continued inflammation the body uses negative feedback mechanisms to stop the inflammatory process. Anti-inflammatory mediators are secreted, pro-inflammatory signals are inhibited, and regulatory cells are activated (Calder, 2010).
The Omega-3 and -6 fatty acids are important in this inflammatory cycle, with the Omega-6 fatty acid Linoleic acid, which is found most abundantly in cereal grains, being involved in the pro-inflammatory processes, and the Omega-3 fatty acids found predominantly in pasture, forage, fish oils and algae, having key roles in resolving inflammation.
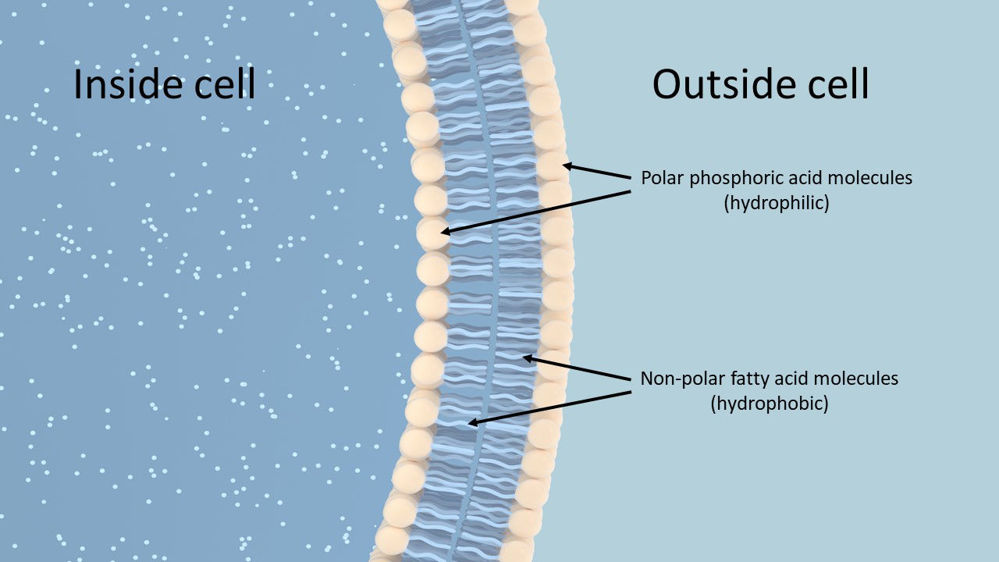
Figure 2. Simplified illustration of a Phospholipid bilayer that makes up cell membranes. The water loving (hydrophilic) phosphoric acid heads make up the outer surfaces of the bilayer, whilst the insoluble, water hating (hydrophobic) fatty acids sit inside the bilayer.
Upon digestion, both Omega-3 and Omega-6 fatty acids are metabolised to longer chain fatty acids containing 20 and 22 carbon atoms. This process requires enzymes known as desaturases which add another double bond to the fatty acid chain, so making the chain less saturated and hence the name desaturases. Further enzymes known as elongases add two carbons to the fatty acid chains, and this is followed by a further desaturation stage. The Omega-6 fatty acid Linoleic (18:2 n-6) is metabolised to Arachidonic acid (20:4 n-6), whilst α-linolenic acid (18:3 n-3) is metabolised to Eicosapentaenoic acid (EPA) (20:5 n-3). Eicosapentaenoic acid can undergo further stages of elongation and desaturation to produce Docosahexaenoic acid (DHA) (22:6 n-3) (Simopoulos, 2016) (Figure 3). The desaturase enzymes have preference for the Omega-3 fatty acid α-linolenic acid however a high intake of Linoleic acid can interfere with desaturation of α-linolenic acid (Simopoulos, 2016).
PRO-INFLAMMATORY PROCESSES
Arachidonic acid is incorporated into phospholipids that comprise cell membranes, including the membranes of leukocytes (macrophages, neutrophils and lymphocytes). Upon injury or irritation, Arachidonic acid is released from cell membranes and is enzymatically converted to eicosanoids which are inflammatory mediators and include prostaglandins, thromboxanes and leukotrienes (Calder, 2010). In addition, leukocytes (white blood cells) travel to the injured site to defend the body against pathogens. The infiltration of blood and leukocytes combined with the release and conversion of Arachidonic acid from cell membranes, results in the swelling we associate with inflammation.
INFLAMMATORY RESOLVING PROCESSES
In contrast to Arachidonic acid, the Omega-3 fatty acids EPA and DHA have anti-inflammatory properties due to their conversion to pro-resolving mediators. Eicosapentaenoic acid and DHA are found in the blood plasma and in cell membranes (Duvall & Levy, 2016). When inflammation develops EPA and DHA flow to the site via the plasma where they are enzymatically converted to the pro-resolving mediators resolvins, protectins and maresins (Duvall & Levy, 2016). Specifically, EPA is converted to E-series resolvins and DHA to D-series resolvins, protectins and maresins. Eicosapentaenoic acid and DHA present in cell membranes can also be released and converted, increasing the levels of pro-resolving mediators. These pro-resolving mediators are part of a cascade of processes that clear the injured site of dead and dying cells and reduce chemical signals that attract leukocytes to the injured site, reducing the pro-inflammatory signals and resolving inflammation (Duvall & Levy, 2016).
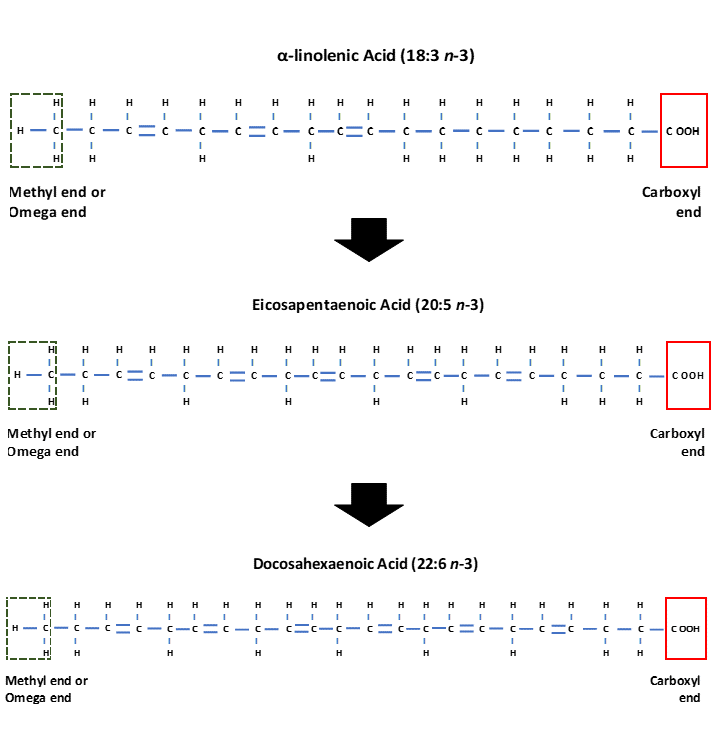
Figure 3. Structure of the Omega-3 fatty acid α-linolenic acid (18:3 n-3) which is enzymatically converted to Eicosapentaenoic acid (EPA) (20:5 n-3) and Docosahexaenoic acid (DHA) (22:6 n-3).
It is evident that both pro-inflammatory and anti-inflammatory processes are essential for maintaining a healthy immune response and protecting the body against disease. Prolonged inflammation however, damages cells and is debilitating for the animal. Both Omega-3 and -6 are important in the diet although studies in humans and animals have shown that the inflammatory response can be partly manipulated through the ratio of Omega-3 to Omega-6 fatty acids in the diet (James et al., 2000), resulting in beneficial effects on the management of certain conditions.
DIETARY SOURCES OF FATTY ACIDS
As shown in tables 1 and 2, cereal grains, corn oil and soya bean oil supply predominantly Linoleic acid (n-6), whilst pasture, forages, linseed oil and rapeseed oil predominantly supply α-linolenic acid (n-3). What is also evident is that plant-based foods are poor sources of Arachidonic acid, EPA and DHA used by the body in the inflammatory cycle, meaning the body must convert Linoleic and α-linolenic acid to these longer chain fatty acids. Conversion of α-linolenic acid in particular is extremely inefficient in mammals (including humans) (Gibson et al., 2013; Ross-Jones et al., 2014; Calder, 2015), therefore the amount of EPA and DHA produced from consuming and metabolising plant sources of α-linolenic acid is low. For many horses this low dietary intake may be sufficient, making linseed and rapeseed products ideal for feeding alongside a pasture/forage-based diet. Horses performing more demanding exercise, breeding stock or those suffering from inflammatory related conditions however may benefit from consuming a dietary supply of EPA and/or DHA to help support the inflammatory-resolving processes (Ross-Jones et al., 2014; Nogradi et al., 2015; Duvall & Levy, 2016). Traditionally the only way of providing a dietary source of these fatty acids was in the form of Cod Liver Oil products as these contained notable amounts of EPA and DHA (Sargent, 1997). Fish oils continue to be popular for supplying EPA and DHA in equine diets, however they are not that palatable to horses (McMillan et al., 2012; Jerina et al., 2021), therefore Green Lipped Mussels (Perna canaliculus) have been used over recent years to supply EPA and DHA.
The high levels of EPA and DHA in fish oils and Green Lipped Mussels are due to these animals feeding on microalgae within the water they live (Miller et al., 2014). These microalgae contain high concentrations of Omega-3 fatty acids which are then found in animals that consume them, with the amount of EPA and DHA within the algae specific to the microalgae species and environment in which it grows (Adarme-Vega et al., 2012). There is now great interest in the opportunities that microalgae provide as dietary sources of Omega-3 fatty acids as they are more sustainable sources than fish oils (Figure 4). When we consider options for feeding to horses, algae align more to the natural vegan diet of equids as algae are classed as thallophytes which are plants lacking in roots, stems and leaves (Lee, 2008). Green Lipped Mussels and fish oils continue to be popular and suitable ingredients for feeding to horses, therefore the choice of which to feed is dependent on owner preference.
HEALTH BENEFITS OF OMEGA-3 FATTY ACIDS
There are multiple health benefits reported in humans attributed to consuming a diet containing more Omega-3 fatty acids compared to Omega-6 fatty acids. Research in animals also shows health benefits although studies have mainly focused on the common health issues affecting domestic species. Specific to horses, research has focused on the effects of feeding fatty acids on joint, respiratory, and skin health, and on their benefits for breeding stock.

Figure 4. Oils containing microalgae now offer a vegan alternative to marine sources of EPA and DHA.
JOINT SUPPORT
Research in humans and dogs has shown that a dietary supply of Omega-3 fatty acids is associated with a reduction in inflammation and/or pain associated with osteoarthritis (Gruenwald et al., 2009; Fritsch et al., 2010), supporting the view that these fatty acids may be beneficial to horses. Woodward et al. (2007) compared the stride length, lameness score, plasma fatty acid concentrations, and inflammatory marker levels of horses fed EPA and DHA for 75 days, with those of horses fed a control diet of corn oil (high in Omega-6). Trot stride length measurements taken on day 75 had increased by 6.6% (~15cm) in horses fed EPA and DHA compared to their measurements at the start of the study, whereas there were no changes in stride length of horses receiving the control diet. The EPA and DHA treatment also resulted in an increase in n-3:n-6 ratio and plasma DHA concentration throughout the study but had no effect on EPA levels. The authors also reported an increase in Arachidonic acid concentrations in both the control and EPA/DHA treatment groups, which was an unexpected result and attributed to the corn oil in the control diet and higher than expected Linoleic and Arachidonic acid levels in the EPA/DHA supplement. Inflammatory markers were not affected by the feeding of EPA and DHA in this study, although this may be due to the presence of Omega-6 fatty acids in the treatment diet which have a pro-inflammatory action. Exercise sessions in the study also varied between horses and treatments, as the horses were used for student riding sessions. As such, some horses may have performed more demanding exercise than others, potentially influencing inflammatory marker levels.
Ross et al. (2010) studied the effect of feeding flaxseed (linseed) and fish oil for 90 days on the presence of EPA and DHA in equine synovial fluid. Synovial fluid extracted from the carpal (knee) joints of horses consuming the fish oil contained EPA and DHA, whereas these fatty acids were undetectable in synovial fluid from horses consuming flaxseed and those in the control group (no supplement). A very similar study was also performed by Ross-Jones et al. (2014) comparing the feeding of flaxseed with a marine supplement (fish oil and algae combined) for 90 days, although the authors measured fatty acid concentration in plasma and synovial fluid, and inflammatory marker (prostaglandin E2 [PGE2]) levels in synovial fluid. As would be expected, feeding flaxseed increased plasma concentrations of α-linolenic acid, however it also increased concentrations of Linoleic acid. The marine supplement increased levels of plasma Arachidonic acid, EPA and DHA compared to the control and flaxseed groups, with EPA and DHA only being detectable in horses receiving the marine supplement. The marine supplement also increased levels of EPA and DHA in synovial fluid, whereas these fatty acids were either undetectable or present in extremely low levels in the synovial fluid of horses consuming flaxseed and the control diets. Synovial PGE2 concentrations did not differ statistically between treatment groups, however there was a trend for PGE2 concentration to be lower in horses consuming the marine supplement compared to horses in the other two groups. The studies by Ross et al. (2010) and Ross-Jones et al. (2014) support the view that α-linolenic acid is not effectively converted to EPA or DHA in equids. The trend for lower PGE2 concentrations in synovial fluid following supplementation with EPA and DHA align with previous results by Manhart et al. (2009) who also reported lower inflammatory markers in the synovial fluid of horses fed EPA and DHA.
Positive results for the feeding of dried Green Lipped Mussel extract on lameness in horses were reported by Cayzer et al. (2012). In a randomised, double-blind, placebo-controlled study, the effect of feeding Green Lipped Mussel extract to horses for 53-59 days on lameness scores, flexion tests and joint pain were compared to the same measurements from horses receiving a cellulose based placebo. None of the lameness scores of the 19 horses in the Green Lipped Mussel group worsened over the study period, with seven horses’ scores remaining the same whilst 12 horses’ scores improved. In comparison, six of the horses in the placebo group recorded a worse lameness score, whilst 11 remained the same and three showed improvements. The improvements in lameness score seen in the supplemented horses were also greater compared to the placebo group. Greater improvements in flexion tests were also seen in the supplemented horses compared to improvements in the control group. Pain measurements were also significantly reduced in the supplemented group. These results indicate that a Green Lipped Mussel extract may be of benefit to horses to support joint function and relieve discomfort due to osteoarthritis, with these results potentially related to the high EPA and DHA content of the mussels.
Studies on the effects of microalgae in horses are limited due to algae being a relatively new ingredient in the equine and animal supplements market. Brennan et al. (2017) compared lameness scores, heart rate and blood cytokine levels (cell signalling molecules) of horses following 60 days of either a microalgae supplement high in DHA (DHA level not provided), or a placebo diet. Following the 60 days of treatment/placebo baseline measurements were taken for each horse, after which acute joint inflammation was simulated through injection of lipopolysaccharide into a single knee joint on each horse. All measurements were repeated 12, 24 and 48 hours after joint injection. Horses who had consumed the microalgae supplement had lower heart rates and lameness scores 12 hours after joint injection compared to the placebo group, although there were no differences between groups at 24 and 48 hours for these measurements. Cytokine levels (signalling molecules interleukin-1β and interleukin-8 which have pro-inflammatory roles) were raised in the placebo group but not in the microalgae supplemented group 12 hours after injection, but not at 24 and 48 hours. Lipopolysaccharide injections are used to stimulate inflammation in joints as a model for acute synovial inflammation due to its short-acting effect, which explains the reduction in cytokine levels and lameness scores 24 and 48 hours after injection in all horses. The absence of any worsening in lameness, or increase in cytokines, 12 hours post injection in horses who had consumed the microalgae supplement, indicate that microalgae is a suitable source of dietary DHA that could support joint function through modification of pro-inflammatory signalling molecules.
RESPIRATORY SUPPORT
Inflammation in the horse’s lower airway is associated with an increase in leukocytes (white blood cells: neutrophils, eosinophils, mast cells) and an excessive accumulation of mucus in the airways (Couëtil et al., 2016). Clinical signs such as coughing, nasal discharge, increased respiratory rate at rest and/or during exercises, and reduced performance are displayed to varying degrees in affected horses. Exposure to non-infectious agents such as fungi, moulds, endotoxins, mites, debris, and ultrafine particles are the principle causes, and any reduction in inflammation and associated clinical signs requires reduction of exposure through management of the horse’s environment. In addition to managing the horse’s environment the feeding of Omega-3 fatty acids may reduce the inflammatory response through a reduction in pro-inflammatory signals and production of pro-resolving mediators as previously described. Horses diagnosed with inflammatory airway disease (IAD) and recurrent airway obstruction (RAO) were fed either a dietary supply of EPA and DHA from microalgae as part of a respiratory supplement, or a placebo, and assessed for their respiratory function alongside samples of blood and bronchoalveolar lavage fluid (BALF) taken from the respiratory tract (Nogradi et al., 2015). Horses were assessed before receiving the supplement/placebo and then again 56 days (8 weeks) later. During the 8-week study period horses were fed a pelleted diet and not permitted any forage (hay). Respiratory scores were significantly improved for all horses, regardless of whether they received the EPA/DHA or placebo treatment, although the improvements were greater in the EPA/DHA treated horses. The improvement in all horses regardless of group is likely due to reduced exposure to dust and irritants present in hay, although improvements were seen quicker in horses receiving the EPA/DHA treatment indicating they were not solely due to hay being removed from the diet. A 10-fold increase in plasma DHA concentration and a decrease in neutrophil percentage in BALF collected from horses receiving the EPA/DHA supplement, support this view and indicate that feeding EPA and/or DHA can help to manage respiratory conditions caused by inflammation.
Results from a recent study by Christmann et al. (2021) offer further support for feeding EPA and/or DHA to horses with inflammatory lower airway conditions, as they found increased DHA and EPA concentrations in plasma and BALF collected from horses receiving a fish-based dietary supply of these fatty acids, compared to samples from horses receiving no supplement (control group). Horses received the diets (EPA/DHA or control) for 90 days and results showed it took until day 60 of the study for the increases in EPA/DHA to be seen, indicating that long-term supplementation of horses with EPA and/or DHA is required for their integration into body fluids, and influence on inflammatory processes, to be seen.
SKIN SUPPORT
Linseed oil and micronised linseed meal are ingredients included in horses’ diets due to their low starch and sugar, and high protein and fat content (NRC, 2007). These nutritional qualities lead to linseed products being fed as a source of low-starch energy and to support skin and hoof health. The fatty acid profile of linseed meal/oil is high in Omega-3 α-linolenic acid and low in Omega-6, which make it particularly helpful for inflammatory issues such as Culicoids hypersensitivity, commonly known as sweet itch. The majority of research into the benefits of feeding Omega-3 fatty acids for skin issues has been performed in humans (Sawada et al., 2021) and dogs and cats (Magalhaes et al., 2021) with few studies performed in horses. Results were favourable in most studies, particularly for skin conditions associated with inflammation. Friberg (1999) compared the effect of feeding 200ml linseed oil or 200ml corn oil to horses with sweet itch in a double-blind study so neither the owners nor those assessing the horses' skin lesions knew which oil was fed to which horse at any time point in the study. All horses consumed both diets in a cross-over design, consuming each individual oil for 6 weeks, with a 6-week period between each oil being fed. Results were based on the number of skin lesions and total skin lesion area. Oil type made no statistic difference in skin lesion numbers or total lesion area, although owners stated that their horse’s skin improved when fed the linseed oil. In contrast, O’Neill et al. (2002) found that after 42 days of supplementation, the area of skin affected by sweet itch was significantly less in horses fed linseed meal compared to those fed a placebo of bran. The same study took skin biopsies from the horses to determine fatty acid profiles but found the addition of linseed meal made no change to fatty acid profiles. The lack of change in skin fatty acids, but decrease in skin reaction, indicates that the potential beneficial effects of feeding linseed may be related to other beneficial properties aside from α-linolenic acid, for example amino acids, or that the low conversion rate of α-linolenic acid to EPA and DHA is still enough to support skin health, but not enough to cause a detectable increase in the levels of these fatty acids in the skin. The use of linseed meal which contains amino acids and not just fatty acids, would also explain the difference in results found between the Friberg et al. (1999) and O’Neill et al. (2002) studies, although further research is needed to understand the benefits of different linseed products to horses with skin issues. Research into the effects of feeding EPA and/or DHA would also be beneficial to determine if a dietary source of these fatty acids would be helpful in managing skin issues in horses.
BREEDING SUPPORT
Docosahexaenoic acid is of particular interest for breeding stallions as spermatozoa contain high levels of PUFA, particularly DHA (Brinkso et al., 2005). As DHA must be supplied within the diet, what the stallion is fed directly influences the fatty acid composition of the sperm plasma membrane (Brinkso et al., 2005). Studies in boars have shown that a high DHA to Omega-6 ratio enhances fertility, whilst a low ratio reduces fertility. The semen of stallions used for artificial insemination (AI) can be affected by the cooling and storage process leading to what is known as cold shock. Cooling of sperm can disrupt the sperm membrane lipids and damage the mitochondria (energy centre) within the sperm leading to loss of sperm motility (ability of sperm to move), viability (number of live sperm) and fertilisation capability (Parks & Graham, 1992). Brinsko et al. (2005) studied the effect of feeding a fish oil supplement high in DHA on stallion sperm motion qualities. Horses received either the fish oil supplement or a control diet (no supplement) for 14 weeks, followed by 14 weeks with no supplement, after which time horses swapped treatments for a further 14 weeks. Baseline semen samples were taken before treatments and then after each 14-week period and analysed for quality and fatty acid content. Samples were assessed as fresh samples, cooled samples which had been stored for 24 hours, cooled samples stored for 48 hours, and frozen then thawed samples. Feeding a dietary source of DHA to the stallions resulted in a considerable increase in sperm concentration within ejaculates and the level of DHA within sperm and semen. Motion characteristics of fresh sperm were not affected by feeding DHA, however sperm moved at a faster speed and in a straighter direction in samples that had been cooled and stored for 24 hours and taken from horses fed the DHA supplement. In samples cooled and stored for 48 hours, sperm from horses fed DHA also had greater total and partial motility which are assessments for the number of sperm moving forwards in a straight line. Positive results for feeding DHA were also seen in samples that were frozen then thawed, a process known to reduce sperm motility. The reduction in motility due to freezing/thawing was less in the DHA supplemented horses than those on the control diet. The authors concluded that feeding a dietary source of DHA could be particularly beneficial for stallions with lower fertility and who produce sperm that do not tolerate cooling and freezing processes. These results are supported by a more recent study by Garmsir et al. (2014) who compared the effects of feeding fish oil, fish oil combined with Thyme, just Thyme, and a control diet (no supplementation) on semen and sperm samples collected from Caspian stallions. This study also found that after 60 days, sperm concentration and ejaculate volume increased with fish oil supplementation (with and without Thyme). Similarly, motility of fresh sperm was unaffected by supplementation however motility, membrane integrity and membrane viability of cooled and stored sperm (24 and 48 hours) was increased after receiving the fish-oil-based supplements for 60 days. Garmsir et al. (2014) drew a similar conclusion to that of Brinsko et al. (2005), that feeding of a dietary source of DHA could be beneficial for stallions with lower fertility and whose sperm do not tolerate cold shock.
SUMMARY
The feeding of fat to horses provides a concentrated form of energy and is able to influence the essential fatty profile within the body. Due to their vital roles within the body, both Omega-3 and -6 fatty acids are essential within the diet, with concentrates and associated oils being high in Omega-6, and forage, linseed and marine derived oils being high in Omega-3. Feeding a diet with a higher Omega-3 to Omega-6 ratio influences the inflammatory processes, with Omega-3 fatty acids EPA and DHA shown to have inflammatory-resolving actions. Such actions make them useful additions to the diets of horses affected by joint, respiratory, and skin inflammation. In addition, dietary sources of DHA could benefit stallions with reduced fertility or cold shock sensitive sperm. Options for supplying EPA and DHA include fish oils, Green Lipped Mussels and microalgae, with the latter offering a vegan source which aligns most closely to the natural equine diet.
If you are searching for a high sepcification vegan DHA source, take a look at Ultromega Supspension™. By working in synergy with the body’s natural processes, Ultromega Suspension™ is a beneficial addition to the diet of all horses. Contains algae, a vegan source of DHA, which aligns with the horse’s natural diet.

REFERENCES
Adarme-Vega, T.C., Lim, D.K.Y., Timmins, M., Vernen, F., Li, Y., & Schenk, P.M. (2012). Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microbial Cell Factories, 11: 96.
Brennan, K.M., Whorf, C., Harris, L.E., & Adam, E. (2017). The effect of dietary microalgae on American Association of Equine Practitioners lameness scores and whole blood cytokine gene expression following a lipopolysaccharide challenge in mature horses. Journal of Animal Science, 95(supplement 4): 166.
Brinsko, S.P., Varner, D.D., Love, C.C., Blanchard, T.L., Day, B.C., & Wilson, M.E. (2005). Effect of feeding a DHA-enriched nutraceutical on the quality of fresh, cooled and frozen stallion semen. Theriogenology, 63: 1519-1527.
Calder, P.C. (2010). Omega-3 Fatty Acids and Inflammatory Processes. Nutrients, 2: 355-374.
Calder, P.C. (2015). Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et Biophysica Acta, 1851: 469-484.
Cayzer, J., Hedderley, D., & Gray, S. (2012). A randomised, double-blinded, placebo-controlled study on the efficacy of a unique extract of green-lipped mussel (Perna canaliculus) in horses with chronic fetlock lameness attributed to osteoarthritis. Equine Veterinary Journal, 44: 393-398.
Christmann, U., Hancock, C.L., Poole, C.M., Emery, A.L., Poovey, J.R., Hagg, C., Mattson, E.A., Scarborough, J.J., Christopher, J.S., Dixon, A.T., Craney, D.J., & Wood, P.L. (2021). Dynamics of DHA and EPA supplementation: incorporation into equine plasma, synovial fluid, and surfactant glycerophosphocholines. Metabolomics, 17: 41.
Couëtil, L.L., Cardwell, J.M., Gerber, V., Lavoie, J-P., Léguillette, R., & Richard, E.A. (2016). Inflammatory Airway Disease of Horses—Revised Consensus Statement. Journal of Veterinary Internal Medicine, 30:503-515.
Duvall, M.G., & Levy, B.D. (2016). DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. European Journal of Pharmacology, 785: 144-155.
Frankel, E.N. (2005). Lipid Oxidation, 2nd Ed. Oily Press, Middlesex, UK.
Friberg, L. (1999). Treatment of Culicoides hypersensitive horses with high-dose n-3 fatty acids: a double-blinded crossover study. Veterinary Dermatology, 10(2): 117-122.
Fritsch, D.A., Allenm T.A., Dodd, C.E., Jewell, D.E., Sixby, K.A., Leventhal, P.S., Brejda, J., & Hahn, K.A. (2010). A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. Journal of the American Veterinary Medical Association, 236(5): 535-539.
Garmsir, A.K., Shahneh, A.Z., Jalali, S.M.A., Nouri, H., & Afshar, M. (2014). Effects of Dietary Thyme (Thymus vulgaris) and Fish Oil on Semen Quality of Miniature Caspian Horse. Journal of Equine Veterinary Science, 34: 1069-1075.
Gibson, R.A., Neumann, M.A., Lien, E.L., Boyd, K.A., & Tu, W.C. (2013). Docosahexaenoic acid synthesis from alpha-linolenic acid is inhibited by diets high in polyunsaturated fatty acids. Prostaglandins, Leukotrienes and Essential Fatty Acids, 88: 139-146.
Glasser, F., Doreau, M., Maxin, G., & Baumont, R. (2013). Fat and fatty acid content and composition of forages: A meta-analysis. Animal Feed Science and Technology, 185: 19-34.
Goodman, S.R. (2021). Cell Membranes. In: Goodman’s Medical Cell Biology, 4th Ed. Academic Press, London, UK.
Gruenwald, J., Petzold, E., Busch, R., Petzold, H-P., & Graubaum, H-J. (2009). Effect of Glucosamine Sulfate with or without Omega-3 Fatty Acids in Patients with Osteoarthritis. Advances in Therapy, 26: 858-871.
Horton, H.R., Moran, L.A., Ochs, R.S., Rawn, J.D., & Scrimgeour, K.G. (1996). Principles of Biochemistry, 2nd Ed. Prentice Hall, USA.
James, M.J., Gibson, R.A., & Cleland, L.G. (2000). Dietary polyunsaturated fatty acids and inflammatory mediator production. American Journal of Clinical Nutrition, 71(supplement): 343S-348S.
Jerina, M.L., Jacobs, R.D., & Gordon, M.E. (2021). Palatability of Ahiflower oil versus flaxseed and fish oil. Journal of Equine Veterinary Science, 100: 103532.
Lee, R.E. (2008). Phycology, 4th Ed. Cambridge University Press, Cambridge, UK.
Magalhaes, T.R., Lourenco, A.L., Gregório, H., & Queiroga, F.L. (2021). Therapeutic Effect of EPA/DHA Supplementation in Neoplastic and Non-neoplastic Companion Animal Diseases: A Systematic Review. In vivo, 35: 1419-1436.
Manhart, D.R., Scott, B.D., Gibbs, P.G., Coverdale, J.A., Eller, E.M., Honnas, C.M., & Hood, D.M. (2009). Markers of Inflammation in Arthritic Horses Fed Omega-3 Fatty Acids. The Professional Animal Scientist, 25(2): 155-160.
Maples, R.D. (2013). Arachidonic Acid Food Sources and Recommendation for the Vegetarian. In: Dumancas, G.G., Murdianti, B.S., & Lucas, E.A. (eds.). Arachidonic Acid: Dietary Sources and General Functions. Nova Biomedical, New York, USA.
McDonald, P., Edwards, R.A., Greenhalgh, J.D.F., Morgan, C.A., Sinclair, L.A. & Wilkinson, R.G. (2011). Animal Nutrition, 7th Ed. Pearson Education Ltd.
McMillan, M.L., Stutts, K.J., Kelley, S.F., Beverly, M.M., & McMillan, L.R. (2012). Effects of Fat Content and Source on Consumption Time in Two-Year-Old Quarter Horses. The Texas Journal of Agriculture and Natural Resources, 25: 24-33.
Miller, M.R., Pearce, L., & Bettjemen, B.I. (2014). Detailed Distribution of Lipids in Greenshell™ Mussel (Perna canaliculus). Nutrients, 6: 1454-1474.
Nogradi, N., Couetil, L.L., Messick, J., Stochelski, M.A., & Burgess, J.R. (2015). Omega-3 Fatty Acid Supplementation Provides an Additional Benefit to a Low-Dust Diet in the Management of Horses with Chronic Lower Airway Inflammatory Disease. Journal of Veterinary Internal Medicine, 29: 299-306.
NRC (2007). Nutrient Requirements of Horses, 6th Ed. The National Academies Press, Washington, USA.
O’Neill, W., McKee, S., & Clarke, A.F. (2002). Flaxseed (Linum usitatissimum) supplementation associated with reduced skin test lesional area in horses with Culicoides hypersensitivity. The Canadian Journal of Veterinary Research, 66: 272-277.
Parks, J.E., & Graham, J.K. (1992). Effects of cryopreservation proceedings on sperm membranes. Theriogenology, 38: 209-222.
Rosentrater, K.A., & Evers, A.D. (2018). Chemical components and nutrition. In: Kent’s Technology of Cereals, 5th Ed. Woodhead Publishing, Duxford, UK.
Ross, T.N., Hess, T.M., Kisiday, J.D., McIlworth, C.W., Engle, T., Hansen,, D.K., & Rexford, J. (2010). Fatty acid composition of synovial fluid in horses fed long chain polyunsaturated fatty acids: A pilot study. Journal of Animal Science, 88(E-supplement): 761.
Ross-Jones, T., Hess, T., Rexford, J., Ahrens, N., Engle, T., & Hansen, D.K. (2014). Effects of Omega-3 Long Chain Polyunsaturated Fatty Acid Supplementation on Equine Synovial Fluid Fatty Acid Composition and Prostaglandin E2. Journal of Equine Veterinary Science, 34: 779-783.
Sargent, J.R. (1997). Fish oils and human diet. British Journal of Nutrition, 78(supplement 1): S5-S13.
Sawada, Y., Saito-Sasaki, N., & Nakamura, M. (2021). Omega 3 Fatty Acid and Skin Diseases. Frontiers in Immunology, 11: 623052.
Schönfeld, P., & Wojtczak, L. (2016). Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. Journal of Lipid Research, 57: 943-954.
Simpoloulos, A.P. (2016). An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients, 128: nu8030128.
Woodward, A.D., Nielsen, B.D., O’Connor, C.L., Skelly, C.D., Webel, S.K., & Orth, M.W. (2007). Supplementation of dietary long-chain polyunsaturated omega-3 fatty acids high in docosahexaenoic acid (DHA) increases plasma DHA concentration and may increase trot stride lengths in horses. Equine and Comparative Exercise Physiology, 4(2): 71-78.

