Dr. Stephanie Wood, PhD Equine Nutrition, PgDip,
BSc (Hons), RNutr (Animal), R.Anim.Tech
Stiffness, lameness, shortened stride and reluctance to move forward are all signs that our horses are not feeling comfortable when they move, and that they are likely to be experiencing some degree of discomfort (Dyson, 2016; Dyson et al., 2020). Whether your horse is retired and spends their day moving around their field, or competing at the highest level, pain-free, easy movement is desired. Achieving this can be challenging, especially when we think about how the horse’s large body frame, lack of muscle in the lower limbs and great athletic ability make their limbs and joints vulnerable to injury and wear and tear. These same factors can also lead to muscle, tendon and ligament issues as a result of repetitive movements, even in those who lead a more sedate lifestyle. Conformational faults, favouring one rein over the other, and poor execution of movements when exercised can also lead to muscle tightness and imbalances, and as a consequence muscle atrophy (wastage) (Dyson, 2016). Providing appropriate management and nutrition combined with a suitable exercise regime will help to maintain and support your horse’s musculoskeletal system and their overall mobility.
MANAGEMENT
Equids have evolved with movement linked to eating (Stephens & Krebs, 1986), taking a few mouthfuls in one area then taking a step or two to move onto a new area. Often our focus is on ensuring our horses satisfy their behavioural and nutritional needs to graze for 12 to 18 hours each day, whilst the need for movement is largely overlooked. Time in the field or turnout area allows free movement and promotes circulation of blood and lymph that transport oxygen and nutrients to the cells and tissues and remove waste material from the body (Frick, 2010). Free movement also activates the musculoskeletal system without the constraints or weight of a rider, helping to maintain fitness and keep bones, joints and muscles metabolically active. Studies comparing the effects of constant stabling, stabling combined with exercise, and constant turnout to pasture, on fitness levels and bone density in horses, repeatedly show that pasture turnout enables horses to maintain their bone density and level of fitness, and that immobility due to constant stabling reduces both of these factors (Nielsen & Spooner, 2008; Graham-Thiers & Bowen, 2009; Logan et al., 2019). Free movement may also help with maintaining insulin sensitivity as low-intensity exercise has been shown to increase the response to insulin in horses and ponies (Bamford et al., 2019), although an earlier study found turnout alone to be insufficient to influence insulin responses (Turner et al., 2011).

As such, the effect of turnout on maintaining fitness and insulin responses will be shaped by how active your horse is when allowed free movement. For horses without any health issues, management regimes should aim to provide frequent and regular opportunities for free movement, although it is recognised that for some animals such opportunities need to be balanced with other requirements. Animals with injuries, or recovering from injury, will often require their movement to be controlled for a period of time to allow recovery, whilst those who are overweight or prone to laminitis will usually have their grass intake controlled which may in some circumstances require restricted turnout. In such circumstances, maintaining mobility is even more challenging although stable management practices such as feeding at floor level which requires horses to stretch their muscles, is a simple practice that supports muscle health whilst also encouraging natural drainage of the respiratory system. Adding stretches to your horse’s routine may also be useful in supporting their mobility.
Maintaining our equids at a healthy weight is also important for their mobility. Excess weight reduces their mobility and performance due to increased strain on their joints, which is magnified in high impact activities such as jumping (Clayton, 1997; Rendle et al., 2019). The more weight travelling through the limbs, the greater the wear and tear on the joints and soft tissues supporting the joints. Research in overweight dogs (Marshall et al., 2010) and humans (Bliddal et al., 2014) with reduced mobility due to limb osteoarthritis showed that a reduction in body weight to a healthy weight, lead to improvements in mobility and a reduction in lameness scores. Jansson et al. (2021) recently demonstrated the effect of greater body fat levels on performance, with horses having greater asymmetry in their movement when their fat stores increased. The same study also confirms that excess body weight increases the work the respiratory and circulatory systems have to perform when horses exercise, leading to a reduction in performance capacity and the likelihood of earlier onset fatigue (Jansson et al., 2021). In view of these findings, prevention of excess body weight in the first instance is ideal, although this can be difficult in horses and ponies that seem to thrive on fresh air and preferentially store fat. The benefits of weight loss on mobility and performance however do show how even a slight decrease in body weight supports joint health.

Figure 1. Performing baited stretches with your horse increases flexibility and range of movement, supporting their overall mobility.
PHYSIOTHERAPY
Some horses may also benefit from physiotherapy to help maintain or improve joint and muscle function. Physiotherapy helps to restore movement and function when an individual is affected by injury, illness or disability, with treatment focusing on restoring painless, optimal function, and prevention of loss of function (Tabor, 2021). For some equids, building regular physiotherapy sessions into their management regime can help them to perform at their best or manage ongoing issues, whereas for others less frequent, periodic sessions are more appropriate. The best option for your horse should be decided through discussions with your vet and physiotherapist who should work collaboratively to support your horse. One activity that is often promoted as suitable for owners to perform with their horses is stretching. Stretching seeks to increase the horse’s flexibility and elasticity with the overall aim of reducing pain and the strain on soft tissues, and increasing joint range of motion (Frick, 2010). Active stretches are where the horse moves its own body through muscle contraction and can be performed during groundwork or when ridden. Baited stretches which use a treat or bait to encourage the horse to stretch to a certain position, are commonly used active stretches, encouraging the horse to stretch its head and neck down or to the side with the aim of stretching the neck and back muscles and engaging the abdominal muscles (Figure 1). Passive stretches are where a person moves the horse’s body for them and is commonly seen in groundwork when the horse’s limbs are stretched forwards. Due to the degree of stretch being decided by the person performing passive stretches, they are not recommended unless training on how to perform them correctly is received from a qualified professional (personal communication – Jessica Seeley, JKG Physiotherapy, UK). Stretching can be performed before exercise, between exercise sessions and after exercise, although stretching routines should be tailored to the purpose of the stretching, and your horse’s specific requirements. Regardless of when stretching takes place, it is important that the muscles are warmed up prior to stretches being performed. Physically warming the tissues increases the extent to which the tissues can stretch, extending how far the stretch can go and helping to reduce the risk of overstretching and damaging the tissues (Frick, 2010). See Role of physiotherapy in supporting mobility.
EXERCISE
Any exercise sessions should be appropriate for the horse and rider’s level of training and fitness to ensure the body can respond to what is asked of it. Preparation for the main exercise activity through appropriate warm-up procedures has been shown to be extremely important in human, canine and equine athletes (McCutcheon et al., 1999; Steiss, 2002; Racinais et al., 2017). The warm-up functions to transition the body from the resting state to an active state and, through this transition, reduce the risk of injury and optimise performance (Steiss, 2002; Pösö et al., 2008). The term warm-up is literal, as muscle activity increases muscle temperature leading to a rise in core body temperature (Racinais et al., 2017). The rise in muscle and core temperature increases elasticity of muscles, tendons and ligaments, and is thought to help prevent injury by increasing support for joints and reducing excessive strain of these soft tissues (Pösö et al., 2008; Racinais et al., 2017). Movement also promotes stretching of muscles and lubrication of joints. The joints of the horse’s limbs are synovial joints that contain a joint cavity filled with lubricating synovial fluid. This fluid is important for preventing friction between the bones within the joint. Flexion of the synovial joints increases pressure within the joint cavity, causing an outflow of synovial fluid from the joint. Extension of the joint reduces pressure leading to an inflow of fluid into the joint cavity (Levick & McDonald, 1995). As such, movement is integral to joint lubrication and health. When movement is limited, either due to being stabled for long periods or transportation, horses can develop filled legs due to the lack of joint flexion and extension, and therefore lack of synovial fluid flow, leading to the fluid pooling in the lower limbs (Bertone, 2008).
The warm-up also triggers responses from the cardiovascular and respiratory systems. Increases in heart rate and stroke volume (volume of blood ejected per beat from the left or right ventricle) increase cardiac output, and the spleen is triggered to contract and release red blood cells (RBC) into the circulation (Poole & Erickson, 2008). Blood vessels serving the muscles dilate whilst those to other organs constrict, leading to preferential blood flow to the skeletal muscles (Pösö et al., 2008). The horse’s respiratory rate increases as does the amount of air inhaled per breath (tidal volume), which when combined with the cardiovascular responses, increase oxygen supplied to the muscles (Ainsworth, 2008). Onset of exercise also triggers cell metabolism to switch from energy storage to energy utilisation (Pösö et al., 2008). These physiological responses to the onset of exercise show how warming up our horses prepares them for more demanding activity. Recommendations for warm-up protocols are lacking as they are specific to the individual horse, activity requirements and environmental conditions. The balance between preparing the body for more demanding exercise without draining energy sources can be challenging, especially in warmer climates as we have seen recently with the Olympics in Tokyo. In addition, having too long a period between the warm-up and onset of more demanding exercise reduces the benefits of the warm-up if the body begins to cool down (Pösö et al., 2008). For horses competing or required to perform specific activities there is also a need to prepare them mentally for the tasks ahead.
The warm-down period is also important for any horse, allowing the body to slowly return to a near resting state. By continuing with low intensity exercise blood continues to circulate, removing the waste products of metabolism from the muscles and lowering body temperature through the dissipation of heat (Steiss, 2002). What activity is performed during the warm-down will again depend on the individual horse, exercise performed and environment. In cooler conditions the focus will be on returning heart and respiratory rates to resting without allowing the horse to become cold, whereas in hot conditions rapid cooling is used to quickly reduce body temperature whilst the horse’s heart and respiratory rate gradually decrease.
NUTRITION
Supporting mobility through nutrition focuses on providing key ingredients in the diet. Ingredients to support joints have been fed for many years, with joint products being the most common supplement fed to horses (Agar et al., 2016; Murray et al., 2018), whereas ingredients specifically to support muscle function, development and recovery are a more recent addition to the equine feed and supplement market. Joint and muscle supporting ingredients are not replacements for correct training or appropriate management, but instead have complementary roles alongside a balanced diet, appropriate management and tailored training and exercise.
JOINT STRUCTURE
To understand how certain ingredients support the horse’s joints we need to understand the structures comprising the joints. The joints we are most often concerned with for our horses are the synovial joints, which generally, but not always, have a wide range of movement and form the pastern, fetlock, knee, elbow, hock, hip and sacroiliac joints. All bone surfaces that articulate have a cartilage covering (Figure 2). In the horse’s limbs this articular cartilage is hyaline cartilage, and its role is to reduce friction between the bones and absorb shock (Dyce et al., 1996; van Weeran & Brama, 2001). Hyaline cartilage is made of cartilage cells (chondrocytes) and an extracellular matrix that acts like a frame structure around the cells. The extracellular matrix is made up of collagen fibrils (mainly type II) (~15%), proteoglycans (~10%) and water (~75%). The collagen fibrils are anchored within calcified cartilage that lies beneath the hyaline cartilage and adjacent to the subchondral bone (van Weeran, 2014), securing the cartilage to the bone. The chondrocyte cells produce the proteoglycans, which consist of a central protein core and one or more glycosaminoglycan (GAG) side chains (van Weeran & Brama, 2001).
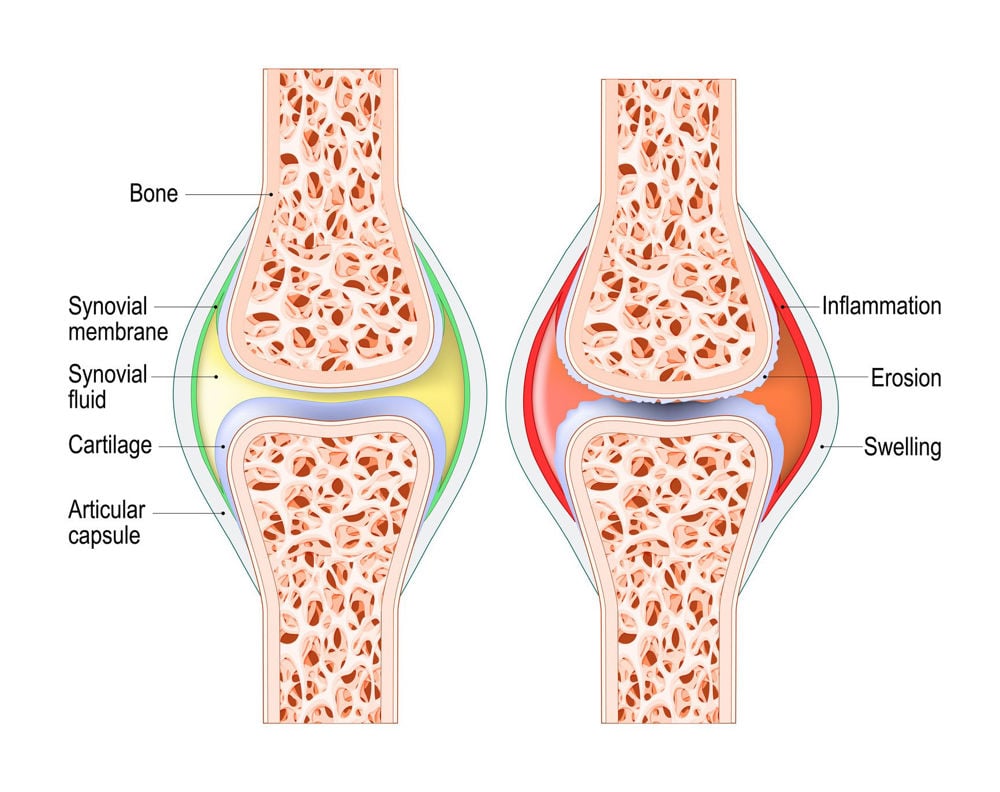
Figure 2. Diagram of a healthy synovial joint (left) and an inflamed synovial joint (right).
The most important proteoglycan in articular cartilage is aggrecan which has a large number of GAG side chains which comprise either Chondroitin sulphate or keratin, with Glucosamine forming part of the keratin (van Weeran & Brama, 2001). Aggrecan molecules attach to Hyaluronic acid (HA), another important proteoglycan produced by chondrocytes (Mahmoud, 2021). Aggrecan connects to the collagen network both directly and via HA molecules. The result of these interlinked complex structures is articular cartilage that is able to withstand stretching (tensile strength) due to collagen, and resist deformation under pressure due to proteoglycans (Bertone, 2008).
Articular cartilage has no blood or nerve supply, and instead receives oxygen and nutrients via diffusion from the synovial fluid, synovial membrane, and joint capsule (Bertone, 2008). This lack of blood supply makes regeneration of articular cartilage almost impossible in adult horses, although there is some capacity for repair in young animals (van Weeran & Brama, 2001). Due to this poor capacity to repair, treatment options for articular cartilage damage are limited. Studies into the effectiveness of stem cell treatment for articular cartilage repair in equids are ongoing, and although there have been some favourable results, this is not currently a routine treatment. As such, reducing articular cartilage degeneration is preferred, with joint supporting ingredients being one method of supporting the cartilage.
The synovial membrane completes the inner lining of the joint capsule, reaching from one articular cartilage to the other (Figure 2). The synovial membrane is only a few cells thick and contains type A cells that have a protective role in the joint, removing cell debris, and type B synoviocytes that synthesise HA (Bertone, 2008; van Weeran, 2014). Both cells are also able to produce molecules involved in inflammation which is associated with common joint diseases such as osteoarthritis (van Weeran, 2014). The synovial membrane contains nerves, capillaries and lymphatic vessels, meaning it provides a pathway for the delivery of oxygen and nutrients to the joint structures, including the articular cartilage, and the removal of waste products (Bertone, 2008). The synovial membranes have a sieve-like action that regulates the molecules entering the synovial fluid. As a result, the molecular size of ingredients fed to support joint function must be small enough to pass through, although molecules up to 10kDa are able to pass from the blood into the joint cavity and synovial fluid (van Weeran, 2014).
Synovial fluid is said to be produced by the synovial membrane, however it is more correctly termed an ultrafiltrate of plasma (van Weeran & Brama, 2001; Bertone, 2008), meaning that blood plasma has been filtered through the synovial membrane to remove high molecular weight proteins. Hyaluronic acid is added to the fluid by synoviocytes to produce a fluid that is highly viscous (thick, sticky) and yellow in colour (van Weeran, 2014). This viscous fluid facilitates smooth movement of the articular cartilages, thus has a lubricating action. It also provides a medium through which oxygen, nutrient and waste product exchanges occur. The composition of the synovial fluid is controlled by the synovial membrane, therefore in cases of joint damage or disease it is likely that substances promoting inflammation (mediators and cytokines) are released by the synovial membrane into the synovial fluid, where they exert their damaging effects on the articular cartilage (van Weeran, 2014).
The joint capsule is a fibrous membrane that forms the outer layer of the joint. Its purpose is to provide support to the joint through attachment to the outer surface of the articulating bones. Ligaments are formed by bundles of this fibrous membrane and provide further strength and support to joints (Dyce et al., 1996).
JOINT SUPPORT
From the review of joint structure, it is easy to see why ingredients such as Glucosamine, Chondroitin sulphate and HA could support joint health, as they are integral to key joint structures. It would be easy to assume that providing a dietary source of these compounds could ‘top-up’ the levels within the joint structures and therefore help to maintain joint function (Laverty et al., 2005). However, the mechanisms of these substances are not that straight forward, with modes of action being related more to slowing the rate of joint degradation through inhibition of cartilage degrading enzymes and inflammatory mediators (Dechant & Baxter, 2007), and not the repair of joint tissues as we remember that articular cartilage cannot regenerate in adult horses.
Glucosamine and Chondroitin sulphate are the most researched joint supporting ingredients although the number of studies using horses as the subject are limited, most likely due to the high cost of performing research on large animals, and the ethical considerations of using animals in research. An alternative to using live animals are in vitro studies in the laboratory, using extracts of equine joint tissues. Fenton et al. (2002) used articular cartilage from horses’ carpal (knee) joints and induced cartilage degradation as a model for osteoarthritis. They tested the effect of three levels of Glucosamine hydrochloride (HCl) on the articular cartilage and found that enzymes associated with degradation of cartilage were reduced by the Glucosamine, with the greatest effects seen at the higher doses. The same study also reported a reduction in nitric oxide and prostaglandin E2 which are both linked to the development of osteoarthritis. Such results show that Glucosamine HCl has a potentially protective action on joint cartilage, however the authors acknowledged that the high Glucosamine dose required to exert an effect in vitro, is unlikely to be suitable for use in the live animal and may even result in cell damage. Neil et al. (2006) also performed an in vitro study using extracted equine cartilage and tested the effectiveness of either Glucosamine or Chondroitin sulphate on the activity of enzymes (matrix metalloproteinases [MMPs], aggrecanases) and inflammatory mediators (nitric oxide, prostaglandin E2) associated with osteoarthritis. They found that Glucosamine was particularly effective in reducing enzyme activity and had a limited effect on reducing inflammatory mediators, however there were no reported effects of Chondroitin sulphate. The authors attributed the lack of effect of Chondroitin sulphate to a too low dose, highlighting the challenge of extrapolating results from in vitro studies to the live animal.
One point for consideration when compounds are administered to the live animal in oral form is the amount of compound absorbed by the animal, known as its bioavailability. A criticism of oral Glucosamine and Chondroitin sulphate given to horses, are their low absorption rates which are approximately 5-9% for Glucosamine and 32% for low molecular weight (8kDa) Chondroitin sulphate (Du et al., 2004; Laverty et al., 2005; Meulyzer et al., 2008). Indeed, a study by Welch et al. (2012) found there to be no increase in Glucosamine or Chondroitin sulphate concentrations in the plasma of six horses fed Glucosamine and Chondroitin sulphate, suggesting that these compounds undergo some form of digestion prior to being absorbed which could influence their effectiveness. It must be highlighted however, that low bioavailability is not necessarily an issue if adequate amounts of a compound are fed to exert an effect. It is also vital that compounds reach the target tissues, which in the case of joint support is the synovial fluid. Increased levels of Glucosamine have been detected in synovial fluid after oral administration of clinically recommended doses to horses (Laverty et al., 2005; Meulyzer et al., 2008). Orally administered Chondroitin sulphate has also been detected in the synovial fluid of dogs, rats (Palmieri et al., 1990) and horses (Moreira et al., 2019). Glucosamine and Chondroitin sulphate are frequently administered in combination to enhance their efficacy (Orth et al., 2002).
Hyaluronic acid and Methyl sulphonyl methane (MSM) are also fed to support joints. As a constituent of articular cartilage and synovial fluid, HA supplementation supports joint function by enhancing synthesis of chondrocytes, HA and proteoglycans and by inhibiting inflammatory mediators and enzymes involved in articular cartilage degradation (Gupta et al., 2019). Hyaluronic acid has been shown to have good bioavailability in rats, dogs and horses (Pierce 2004; Balogh et al., 2008) and to reach articular cartilage and synovial fluid. Methyl sulphonyl methane is a sulphur-containing compound fed for its antioxidant and anti-inflammatory effects (Butawan et al., 2020). The body readily uses the sulphur from MSM in connective tissue, and it is also thought to contribute to articular cartilage structure, although the absorption of MSM varies with species. Methyl sulphonyl methane has been shown to reduce pain associated with osteoarthritis by blocking the pain response in nerve fibres (Gupta et al., 2021). The majority of research on MSM has focused on humans however the antioxidant, anti-inflammatory, and analgesic properties make it a common addition to horses’ diets, either individually or in combination with the previously mentioned joint supporting ingredients.
Studies assessing the efficacy of joint supplements for supporting movement in live horses have shown variable results. Comparison between studies is difficult due to different study designs and durations, the use of horses with healthy joints, artificially induced joint disease, and those with naturally occurring joint disease, the use of different ingredients and feeding levels, and different assessment parameters. Studies published in the 90’s feeding Glucosamine and Chondroitin sulphate to horses used measurements of joint flexion, lameness score, stride length and x-rays to determine the supplement efficacy. White et al. (1994) found no difference in these parameters between supplemented and control horses, whereas Hanson et al. (1997) feeding Glucosamine and Chondroitin sulphate to 25 horses reported a significant increase in joint flexion and stride length, reduction in lameness score after two weeks of receiving the supplement, and a further improvement in lameness score after four weeks of supplementation. This study however did not include a control group for comparison. Dorna & Guerrero (1998) feeding just Chondroitin sulphate, also reported favourable results with supplemented horses having greater joint flexibility and stride length and reduced lameness scores after supplementation for 30 days, compared to horses in the control group.
A longitudinal study by Rogers (2006) used the number of HA and steroid injections required in horses’ joints as a measure of how effective an oral Glucosamine and Chondroitin sulphate supplement was at maintaining mobility. Ten horses received the supplement for six years and the number of joint injections required was monitored over this period. The number of injections over the study period were compared with the number required in the two years prior to the study, with results showing the average number of injections required by horses was less when consuming the supplement. Monitoring horse responses to supplementation over a prolonged time is commendable, however mobility and joint health could have been affected by multiple factors (workload, age, medication) which were not monitored or recorded, therefore attributing a reduction in joint injections and maintenance of mobility to the supplement alone is inappropriate.
A weakness of these early studies is that the people performing the assessments were aware of which horses had been supplemented, and so were not blinded to treatment being tested. This lack of blinding may have influenced assessments of mobility and therefore results gained. A further weakness of the studies by Hanson et al. (1997) and Rogers (2006) was the lack of control group which would have allowed comparison with animals that had not received the treatment, and therefore greater confidence that the results gained were attributed to the treatment. More recently blinded studies have been undertaken to determine the effect of joint supplements on mobility which is a positive step forwards for equine science. Murray et al. (2014) reported improvements in lameness scores assessed in a straight line and on a circle in mature horses administered a supplement containing Chondroitin sulphate, Glucosamine, Vitamin C, MSM and the long chain fatty acid Docosahexaenoic acid (DHA). This study also used the more objective assessment of joint flexion using high speed video gait analysis and reported a significant increase in hock flexion in horses consuming the supplement. Forsyth et al. (2006) had previously used this technology to assess the effect of a Glucosamine and Chondroitin sulphate supplement fed at manufacturer levels to older horses (15-35 years), on gait parameters. Elbow, stifle and hind fetlock joint range of motion increased significantly in supplemented horses after eight weeks and continued until the end of the trial (week 12). There were also trends for greater range of motion in knee, hock and front fetlock joints, although these were not statistically significant. Supplementation also increased stride length by week eight, which continued until the end of the trial. In contrast, Higler et al. (2014) assessing the effect of a Glucosamine, Chondroitin sulphate and MSM supplement on older horses (25-33 years), found no difference between the stride length, knee flexion, fetlock extension and hock range of motion of horses supplemented for three months and those receiving a placebo for the same amount of time.
Differences in results highlight the difficulties of assessing supplement efficacy and standardising trials. Studies clearly show that joint supporting ingredients have effects on joint tissues when tested in vitro, but more research is needed to conclusively determine their effect in the live animal. Whilst research is ongoing the decision on whether to feed joint supplements is up to the individual horse owner. Another area where there is evidence to support beneficial effects on mobility is the use of substances with anti-inflammatory properties such as Devil’s Claw (Axmann et al., 2019), Boswellia (Reichling et al., 2004; Sengupta et al., 2008 – see Ingredient spotlight: Boswellia in JEN issue 1) and Omega-3 fatty acids. Chronic inflammation is known to be a major contributor to joint degeneration therefore feeding ingredients that can counteract inflammation can be beneficial, with such anti-inflammatory effects also being beneficial to ligament, tendon and muscle health.
MUSCLE SUPPORT
The foundations for muscle development and effective function are providing appropriate nutrients for muscles to utilise, and activation of muscles through appropriate exercise. The diet is therefore key and must provide energy for muscle contraction and cellular activity, protein for development and repair of muscle tissue, and micronutrients (vitamins and minerals) for enzymatic and metabolic processes. The high protein level in muscles can lead to a focus on dietary protein supply, when the focus should be on providing a diet that meets all nutrient requirements. It is correct that horses performing more exercise (intensity and/or duration) have higher crude protein requirements than horses with lower exercise demands due to increased muscle development, tissue repair and nitrogen losses in sweat (NRC, 2007; Pratt-Phillips & Lawrence, 2014). However simply feeding more protein may not be the answer and feeding protein in excess of requirements has no additional benefits in exercising horses (Pratt-Phillips & Lawrence, 2014).
Figure 3. The feeding of certain ingredients can help to support muscle function and recovery and can be particularly beneficial to horses performing more strenuous exercise.
The amino acid content of the protein fed is important, with protein containing essential amino acids (amino acids that cannot be made by the horse) classed as high-quality protein. Lysine is the first limiting amino acid meaning it is the amino acid most likely to be deficient in the diet and that limits utilisation of other amino acids (McDonald et al., 2011). Lysine requirements rise with increasing exercise demand. If the diet contains protein sources with low Lysine content, then more of the protein source will need to be fed to meet Lysine requirements. The requirement for other essential amino acids is also likely to increase with higher exercise demands, although these requirements have not been quantified at present. Soya bean meal, Canola meal, legumes and Spirulina are good sources of Lysine and other essential amino acids and would be classed as providing quality protein.
The need for a balanced diet that meets protein and Lysine requirements does not mean that specific ingredients have no place in supporting muscle function. Certain ingredients can help your horse’s muscles, and therefore their mobility and performance. Such ingredients focus on supporting energy metabolism and muscle function, reducing muscle damage, and aiding muscle recovery (Figure 3).
Ingredients aimed at improving energy metabolism within muscles are popular as fatigue is associated with a depletion in energy substrate (mainly associated with long-duration low intensity exercise) or accumulation of lactic acid and other metabolic by-products during high intensity exercise. Such responses cause a decrease in muscle pH which negatively affects muscle contraction and key enzymes involved in utilisation of glycogen (Pösö et al., 2008). Creatine is an ingredient that is often promoted as able to maintain energy supply to the muscles due to its role in energy synthesis at the initial onset of exercise, and its ability to buffer (neutralise) lactic acid (Pratt-Phillips & Lawrence, 2014; Urschel & McKenzie., 2021). Creatine is found in skeletal muscle and is consumed by humans in meat products and oral supplements. Oral Creatine supplementation in people has been shown to increase muscle Creatine concentrations (Cooper et al., 2012), however supplementation of horses has shown no significant increases on muscle Creatine concentrations or performance (Schuback et al., 2000; D’Angelis et al., 2005; Teixeira et al., 2016). Creatine is poorly absorbed by equids as they have evolved consuming a vegan diet, and as a result it is thought that they lack the ability to transport Creatine from the digestive tract (Pratt-Phillips & Lawrence, 2014).
L-Carnitine is another ingredient often promoted as supporting muscle function and performance as it is required for aerobic metabolism of fatty acids (Pratt-Phillips & Lawrence, 2014; Urschel & McKenzie, 2021). Aerobic metabolism is the predominant metabolic pathway during endurance-type exercise, therefore providing additional Carnitine may increase aerobic capacity and overall performance. Studies on people consuming Carnitine produce conflicting results with some supporting its use for increasing aerobic capacity, whilst others found no influence on athletic performance (Gnoni et al., 2020). Oral supplementation of horses with Carnitine showed an increase in plasma Carnitine concentration, however there was no evidence that Carnitine reached the muscles (Harris et al., 1995). The same study also showed a low bioavailability (7%) for oral Carnitine supplementation. Rivero et al. (2002) also studied the effect of oral Carnitine supplementation to horses in training and found that supplemented horses showed improved muscle adaptations to training, but that such adaptations only continued whilst the horses were in training despite continued feeding of Carnitine. The authors concluded that Carnitine supplementation enhances adaptations to training but does not actually cause the changes in muscle tissue per se, therefore feeding Carnitine is only beneficial when horses are performing appropriate exercise.
β-Hydroxy-β-methylbutyrate (HMB) is a metabolite (end product of metabolism) of the amino acid Leucine, meaning it is naturally occurring in the body although in low concentrations (Szcześniak et al., 2015). In vitro and in vivo studies have shown that HMB can stimulate muscle protein synthesis in humans and animals (Smith et al., 2005; Szcześniak et al., 2015; Reister et al., 2017), and as a result is fed to promote muscle development in food producing animals and taken as an oral supplement by people wanting to maintain muscle mass. Along with Leucine, HMB regulates skeletal muscle protein turnover and increases lean body mass and muscle strength.

Supplementation also suppresses muscle breakdown and inhibits cell death. Ostaszewski et al. (2012) studied the effect of oral HMB supplementation on Thoroughbred horses in race training. Supplemented horses had significantly lower post-exercise creatine kinase activity and lactate dehydrogenase concentrations than the placebo group. Creatine kinase and lactate dehydrogenase are enzymes produced when muscle tissues are damaged therefore HMB was shown to be effective in reducing muscle damage.
Supplementation with certain amino acids has also been associated with muscle development and recovery. Leucine, Isoleucine and Valine are all essential amino acids so must be provided in the diet. They are also classed as branched-chain amino acids (BCAA) which are amino acids with a branch off to one side of their structure. Branched-chain amino acids are associated with increased performance due to their effect on prolonging time to fatigue and contribution to energy sources used during exercise. Central fatigue is when there is decreased motivation to perform exercise and a loss of motor coordination and is associated with increased production of Serotonin in the brain (Farris et al., 1998). Tryptophan is used for the synthesis of Serotonin in the brain, therefore high Tryptophan concentrations enable more Serotonin synthesis. Branched-chain amino acids compete with Tryptophan for binding sites at the blood-brain barrier, therefore a higher plasma concentration of BCAA reduces uptake of Tryptophan by the brain leading to a consequential reduction in Serotonin synthesis and onset of central fatigue which reduces performance (Pratt-Phillips & Lawrence, 2014). Such an effect has been reported in horses, with intravenous administration of Tryptophan significantly increasing plasma Tryptophan concentrations which were associated with a reduced performance capacity compared to non-supplemented horses (Farris et al., 1998). Branched-chain amino acids are sometimes referred to as providing an energy source for use during exercise however their direct contribution to energy sources is thought to only be small (Arfuso et al., 2019). They are thought however to contribute indirectly to energy supplies and therefore prolonging exercise, through their oxidation. Oxidation of BCAA in the muscles produces the amino acid Alanine which can then be converted to glucose through the process of gluconeogenesis in the liver (Trottier et al., 2002). The enzymes involved in the breakdown of BCAA in skeletal muscles are found more in type 1 muscle fibres which are associated with aerobic metabolism and prolonged, low intensity exercise. As such, BCAA supplementation, specifically Leucine, could be beneficial for horses performing such exercise (Trottier et al., 2002; Urschel et al., 2010).
Branched-chain amino acids may also facilitate recovery after exercise, particularly exercise that depletes muscle glycogen stores. Replenishing glycogen levels in equine muscles is much slower than in other species and in humans, taking approximately 72 hours (Pratt-Phillips & Lawrence, 2014). Horses required to perform exercise on consecutive days may therefore suffer from low energy levels due to low muscle glycogen stores. Even when feeding meals containing soluble CHOs (starch and sugar), glycogen replenishment in muscles is slow. One proposed reason for this is that equids do not show the increased sensitivity to insulin after exercise that is seen in humans and other species (Pratt et al., 2007). Insulin is needed to promote storage of glucose as glycogen, therefore this lack of response likely contributes to the slow muscle glycogen replenishment. Urschel et al. (2010) found that administering Leucine to resting and exercising horses increased plasma Leucine concentrations in all horses. Leucine is known to stimulate insulin secretion in horses, which was evident in the study by Urschel et al. (2010) as plasma insulin and glucose concentrations were significantly raised post Leucine supplementation. This response to Leucine is a potential method of stimulating glycogen storage in horses without having to feed a high starch or sugar meal. Etz et al. (2011) also reported an increase in plasma Leucine concentration in response to consuming Leucine, however they did not report any effect of supplementation on insulin and glucose plasma levels. The same authors also reported considerable variation in results between horses, suggesting that some horses could benefit from supplementation whilst others will not. It must also be highlighted that Casini et al. (2000) and Stefanon et al. (2000) did not report any significant effects of administering BCAA to exercising horses.
Supplementation with BCAA is also reported to promote protein synthesis post-exercise (Jacobs et al., 2015; Hauss et al., 2021), therefore supporting muscle recovery and mobility. Supplementation with antioxidants offers further support for muscle recovery through their ability to reduce the production of reactive oxygen species (ROS), reactive nitrogen species (RNS) and free radicals (known collectively as oxidants) during exercise and scavenge any oxidants that are produced (Lykkesfeldt & Svendsen, 2007). Oxidants damage cell membranes and structures and therefore affect cell function (Kirschvink et al., 2008). Vitamin E, MSM, Coenzyme Q10 and various herbs are all promoted to have potent antioxidant properties (Williams, 2008; Sinatra et al., 2013; Tanvir et al., 2017; Butawan et al., 2020) although further research is needed to support some of these ingredients.
The final ingredients to highlight for supporting your horse’s muscles are electrolytes which may not always be thought of for muscle function. However, they are integral for normal muscle and nerve function and therefore should be replenished in horses that produce any considerable amount of sweat, regardless of their workload. For those performing work at lower levels, regular table salt (sodium chloride) can be fed daily at a rate of 3-10g per 100kg of body weight, as sodium and chloride are the two main electrolytes lost in sweat (Marlin & Nankervis, 2002). For those producing greater amounts of sweat an electrolyte product is likely to be more applicable.
SUMMARY
Supporting your horse’s mobility is a holistic combination of correct management, training and diet supported by therapeutic and veterinary treatments when required. Only focusing on one of these elements will likely limit any benefits on mobility. The benefits of physiotherapy are highlighted although you may find an alternative therapy is more suited to your horse’s specific requirements. What is important is that you use qualified, certified professionals to undertake such treatments, as they not only have the knowledge to support your horse’s health and performance but will also have the appropriate insurance should it be required. The review of ingredients to support mobility is not exhaustive but provides an overview of the more common ingredients fed. The use of such ingredients should be in the context of a balanced diet that is specific to your horse’s requirements.
REFERENCES
Agar, C., Gemmill, R., Hollands, T., & Freeman, S.L. (2016). The use of nutritional supplements in dressage and eventing horses. Veterinary Record Open, 3: e000154.
Ainsworth, D.M. (2008). Lower airway function: responses to exercise and training. In: Hinchcliff, K.W., Geor, R.J., & Kaneps, A.J. (eds) Equine Exercise Physiology. Saunders Elsevier, London, UK.
Arfuso, F., Assenza, A., Fazio, F., Rizzo, M., Giannetto, C., Piccione, G. (2019). Dynamic Change of Serum Levels of Some Branched-Chain Amino Acids and Tryptophan in Athletic Horses After Different Physical Exercises. Journal of Equine Veterinary Science, 77: 12-16.
Axmann, S., Hummel, K., Nöbauer, K., Razzazi-Fazeli, E., & Zitterl-Eglseer, K. (2019). Pharmacokinetics of harpagoside in horses after intragastric administration of a Devil’s claw (Harpagophytum procumbens) extract. Journal of Veterinary Pharmacology and Therapeutics, 42: 37-44.
Balogh, L., Polyak, A., Mathe, D., Kiraly, R., Thuroczy, J., Terez, M., Janoki, G., Ting, Y., Bucci, L.R., & Schauss, A.G. (2008). Absorption, Uptake and Tissue Affinity of High-Molecular-Weight Hyaluronan after Oral Administration in Rats and Dogs. Journal of Agricultural and Food Chemistry, 56: 10582-10593.
Bamford, N.J., Potter, S.J., Baskerville, C.L., Harris, P.A., & Bailey, S.R. (2019). Influence of dietary restriction and low-intensity exercise on weight loss and insulin sensitivity in obese equids. Journal of Veterinary Internal Medicine, 32: 280-286.
Bertone, A.L. (2008). Joint physiology: responses to exercise and training. In: Hinchcliff, K.W., Geor, R.J., & Kaneps, A.J. (eds) Equine Exercise Physiology. Saunders Elsevier, London, UK.
Bliddal, H., Leeds, A.R., & Christensen, R. (2014). Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons – a scoping review. Obesity Reviews, 15: 578-586.
Butawan, M., Benjamin, R.L., & Bloomer, R.J. (2020). Methylsulfonylmethane as an antioxidant and its use in pathology. In: Preedy, V.R. (ed) Pathology: Oxidative Stress and Dietary Antioxidants. Academic Press, London, UK.
Casini, L., Gatta, D., Magni, L., & Colombani, B. (2000). Effect of prolonged branched-chain amino acid supplementation on metabolic response to anaerobic exercise in Standardbreds. Journal of Equine Veterinary Science, 20(2): 120-123.
Clayton, H.M. (1997). Effect of added weight on landing kinematics in jumping horses. Equine Veterinary Journal, supplement 23: 50-53.
Cooper, R., Naclerio, F., Allgrove, J., & Jimenez, A. (2012). Creatine supplementation with specific view to exercise/sports performance: an update. Journal of the International Society of Sports Nutrition, 9: 33.
D’Angelis, F.H.F., Ferraz, G.C., Boleli, I.C., Lacerda-Neto, J.C., & Queiroz-Neto, A. (2005). Aerobic training, but not creatine supplementation, alters the gluteus medius muscle. Journal of Animal Science, 83: 579-585.
Dechant, J.E., & Baxter, G.M. (2007). Glucosamine and chondroitin sulphate as structure modifying agents in horses. Equine Veterinary Journal, 19(2): 90-96.
Dorna, V., & Guerrero, R.C. (1998). Effects of oral and intramuscular use of chondroitin sulphate in induced equine aseptic arthritis. Journal of Equine Veterinary Science, 18: 548-555.
Du, J., White, N., Eddington, N.D. (2004). The bioavailability and pharmacokinetics of glucosamine hydrochloride and chondroitin sulfate after oral and intravenous single dose administration in the horse. Biopharmaceutics & Drug Disposition, 25(3): 109-116.
Dyce, K.M., Sack, W.O., & Wensing, C.J.G. (1996). Textbook of Veterinary Anatomy. W.B. Saunders Company, Pennsylvania, USA.
Dyson, S. (2016). Evaluation of poor performance in competition horses: A musculoskeletal perspective. Part 1: Clinical assessment. Equine Veterinary Education, 28(5): 284-293.
Dyson, S., Routh, J., Bondi, A., & Pollard, D. (2020). Gait abnormalities and ridden horse behaviour in a convenience sample of the United Kingdom ridden sports horse and leisure horse population. Equine Veterinary Education, doi: 10.1111/eve.13395
Etz, L.C., Lambert, N.M., Sylvester, J.T., Urschel, K.L., & Staniar, W.B. (2011). Supplemental leucine's influence on plasma glucose, insulin, and amino acid responses in Quarter Horse yearlings. Journal of Equine Veterinary Science, 31: 248-249.
Farris, J.W., Hinchcliff, K.W., McKeever, K.H., Lamb, D.R., & Thompson, D.L. (1998). Effect of tryptophan and of glucose on exercise capacity of horses. Journal of Applied Physiology, 85(3): 807-816.
Fenton, J.I., Chlebek-Brown, K.A., Caron, J.P., & Orth, M.W. (2002). Effect of glucosamine on interleukin-1-conditioned articular cartilage. Equine Veterinary Journal, supplement 34: 219-223.
Forsyth, R.K., Briden, C.V., & Northrop, A.J. (2006). Double blind investigation of the effects of oral supplementation of combined glucosamine hydrochloride (GHCL) and chondroitin sulphate (CS) on stride characteristics of veteran horses. Equine Veterinary Journal, supplement 36: 622-625.
Frick, A. (2010). Stretching Exercises for Horses: Are They Effective? Journal of Equine Veterinary Science, 30(1): 50-59.
Gnoni. A., Longo, S., Gnoni, G.V., & Guidetti, A.M. (2020). Carnitine in Human Muscle Bioenergetics: Can Carnitine Supplementation Improve Physical Exercise? Molecules, 25: 182.
Graham-Thiers,P.M., & Bowen, L.K. (2009). Ability to Maintain Fitness Improved during Large Pasture Turnout. Journal of Equine Veterinary Science, 29(5): 430-432.
Gupta, R.C., Kalidindi, S.R., Doss, R.B., Lall, R., Srivastava, A., & Sinha, A. (2021). Nutraceuticals in arthritis. In: Gupta, R.C., Lall, R., & Srivastava, A. (eds) Nutraceuticals: Efficacy, Safety and Toxicity (2nd Ed.). Academic Press, London, UK.
Gupta, R.C., Lall, R., Srivastava, A., & Sinha, A. (2019). Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Frontiers in Veterinary Science, 6: 192.
Hanson, R.R., Smalley, L.R., Huff, G.K., White, S., & Hammond, T.A. (1997). Oral Treatment With a Glucosamine-Chondroitin Sulfate Compound for Degenerative Joint Disease in Horses: 25 Cases. Equine Practice, 19(9): 16-20.
Harris, R.C., Foster, C.V.L., & Snow, D.H. (1995). Plasma carnitine concentration and uptake into muscle following oral and intravenous administration. Equine Veterinary Journal, supplement 18: 382-387.
Hauss, A., Loos, C., Gerritsen, A., Urschel, K., & Pagan, J. (2021). Effect of branched-chain amino acid and N-acetylcysteine supplementation post-exercise on muscle mTOR signalling in exercising horses. Journal of Equine Veterinary Science, 100: 103524.
Higler, M.H., Brommer, H., L’Ami, J.J., de Grauw, J.C., Nielen, M., van Weeran, P.R., Laverty, S., Barnveld, A., & Back, W. (2014). The effects of three-month oral supplementation with a nutraceutical and exercise on the locomotor pattern of aged horses. Equine Veterinary Journal, 46: 611-617.
Jacobs, R.D., Splan, R.K., Urschel, K.L., Mastellar, S., & Wagner, A.L. (2015). Post-exercise dietary supplementation leads to improved muscle recovery in fatigued horses. Journal of Equine Veterinary Science, 35: 395-396.
Jansson, A., Gunnarsson, V., Ringmark, S., Ragnarsson, S., Söderroos, D., Ásgeirsson, E., Jöhannsdóttir, T.R., Liedberg, C., & Stefánsdóttir, G.J. (2021). Increased body fat content in horses alters metabolic and physiological exercise response, decreases performance, and increases locomotion asymmetry. Physiological Reviews, 9: e14824.
Kirschvink, N., de Moffarts, B., & Lekeux, P. (2008). The oxidant/antioxidant equilibrium in horses. The Veterinary Journal, 177(2): 178-191.
Laverty, S., Sandy, J.D., Celeste, C., Vachon, P., Marier, J-F., & Plaas, A.H.K. (2005). Synovial Fluid Levels and Serum Pharmacokinetics in a Large Animal Model Following Treatment With Oral Glucosamine at Clinically Relevant Doses. Arthritis & Rheumatism, 52(1): 181-191.
Levick, J.R., & McDonald, J.N. (1995). Fluid movement across synovium in healthy joints: role of synovial fluid macromolecules. Annals of the Rheumatic Diseases, 54: 417-423.
Logan, A.A., Nielsen, B.D., Sehl, R., Jones, E., Robinson, C.I., Pease, A.P. (2019). Short-term stall housing of horses results in changes of markers of bone metabolism. Comparative Exercise Physiology, 15(4): 283-290.
Lykkesfeldt, J., & Svendsen, O. (2007). Oxidants and antioxidants in disease: Oxidative stress in farm animals. The Veterinary Journal, 173: 502-511.
Mahmoud, E.E., Hassaneen, A.S.A., Noby, M.A., Mawas, A.S., & Abdel-Hady, A-N.A. (2021). Equine osteoarthritis: An overview of different treatment strategies. SVU-International Journal of Veterinary Sciences, 4(2): 85-96.
Marlin, D., & Nankervis, K. (2002). Equine Exercise Physiology. Blackwell Science, Oxford, UK.
Marshall, W.G., Hazewinkel, H.A.W., Mullen, D., De Meyer, G., Baert, K., & Carmichael, S. (2010). The effect of weight loss on lameness in obese dogs with osteoarthritis. Veterinary Research Communications, 34: 241-253.
McCutcheon, L.J., Geor, R.J., & Hinchcliff, K.W. (1999). Effects of prior exercise on muscle metabolism during sprint exercise in horses. Journal of Applied Physiology, 87(5): 1914-1922.
McDonald, P., Edwards, R.A., Greenhalgh, J.D.F., Morgan, C.A., Sinclair, L.A. & Wilkinson, R.G. (2011). Animal Nutrition, 7th Ed. Pearson Education Ltd.
Meulyzer, M., Vachon, P., Beaudry, D., Vinardell, T., Beauchamp, R.G., & Laverty, S. (2008). Comparison of pharmacokinetics of glucosamine and synovial fluid levels following administration of glucosamine sulphate or glucosamine hydrochloride. Osteoarthritis and Cartilage, 16: 973-979.
Moreira, J.J., Coelho, J.M., Sodré, T., Machado, L., Morais, A.P.L., Michelacci, Y.M., & Baccarin, R.Y. A. (2019). Oral glucosamine and chondroitin sulfate on synovial fluid biomarkers from osteoarthritic equine joints. Ciência Rural, 49(9): doi.org/10.1590/0103-8478cr20180247
Murray, J.M.D., Hanna, E., & Hastie, P. (2018). Equine dietary supplements: an insight into their use and perceptions in the Irish equine industry. Irish Veterinary Journal, 71: 4.
Murray, R., Walker, V., Tranquille, C., Adams, V & Frost, R. (2014). Effect of an oral joint supplement on orthopaedic evaluation scores and limb kinematics. In: Proceedings of International Conference on Equine Exercise Physiology, 46: 44-45.
Neil, K.M., Orth, M.W., Coussens, P.M., Chan, P-S., & Caron, J.P. (2005). Effect of glucosamine and chondroitin Sulphate on mediators of osteoarthritis. AAEP Proceedings, 52: 572-573.
Nielsen, B.D., & Spooner, H.S. (2008). Small changes in exercise, not nutrition, often result in measurable changes in bone. Comparative Exercise Physiology, 5(1): 15-20.
NRC (2007). Nutrient Requirements of Horses, 6th Ed. The National Academies Press, Washington, USA.
Orth, M.W., Peters, T.L., & Hawkins, J.N. (2002). Inhibition of articular cartilage degradation by glucosamine-HCl and chondroitin sulphate. Equine Veterinary Journal, supplement 34: 224-229.
Ostaszewski, P., Kowalska, A., Szarska, E., Szpotański, P., Cywinska, A., Balasińska, B., & Sadkowski, T. (2012). Effects of b-Hydroxy-b-Methylbutyrate and g-Oryzanol on Blood Biochemical Markers in Exercising Thoroughbred Race Horses. Journal of Equine Veterinary Science, 32: 542-551.
Palmieri, L., Conte, A., Giovannini, L., Lualdi, P., & Ronca, G. (1990). Metabolic fate of exogenous chondroitin sulfate in the experimental animal. Arzneimittelforschung, 40(3): 319–23.
Pierce, S.W. (2004). Efficacy of orally administered sodium hyaluronate gel in the racing thoroughbred. In: Balazs, E.A., & Hascall, V.C. (eds). Hyaluronan. Chapter 6. Musculoskeletal System. Matrix Biology Institute, Cleveland, USA.
Poole, D.C., & Erickson, H.H. (2008). Cardiovascular function and oxygen transport: responses to exercise and training. In: Hinchcliff, K.W., Geor, R.J., & Kaneps, A.J. (eds) Equine Exercise Physiology. Saunders Elsevier, London, UK.
Pösö, A.R., Hyyppä, S., & Geor, R.J. (2008). Metabolic responses to exercise and training. In: Hinchcliff, K.W., Geor, R.J., & Kaneps, A.J. (eds) Equine Exercise Physiology. Saunders Elsevier, London, UK.
Pratt, S.E., Geor, R.J., Spriet, L.L., & McCutcheon, L.J. (2007). Time course of insulin sensitivity and skeletal muscle glycogen synthase activity after a single bout of exercise in horses. Journal of Applied Physiology, 103: 1063.
Pratt-Phillips, S.E., & Lawrence, L.M. (2014). Nutrition of the Performance Horse. In: Hodgson, D.R., McKeever, K.H., & McGowan, C.M. (eds) The Athletic Horse: Principles and Practice of Equine Sports Medicine (2nd Ed.). Saunders Elsevier, London, UK.
Racinais, S., Cocking, S., & Périad, J. (2017). Sports and environmental temperature: From warming-up to heating-up. Temperature, 4(3): 227-257.
Reichling, J., Schmökel, H., Fitzi, J., Bucher, S., & Saller, R. (2004). Dietary support with Boswellia resin in canine inflammatory joint and spinal disease. Schweiz Arch Tierheilkd, 146(2): 71-79.
Reiter, A.S., DeBoer, M.L., Martinson, K.L., & Hathaway, M.R. (2017). Effect of b-hydroxy-b-methylbutyrate on protein synthesis of cultured equine myogenic satellite cells. Journal of Equine Veterinary Science, 76: 72.
Rendle, D., Austin, C., Bowen, M., Cameron, I., Furtado, T., Hodgkinson, J., McGorum, B., & Matthews, J. (2019). Equine de-worming: a consensus on current best practice. UK-Vet Equine, 3(1): 1-14.
Rivero, J,-L.L., Sporleder, H.-P., Quiroz-Rothe, E., Vervuert, I., Coenan, M., & Harmeyer, J. (2002). Oral L-carnitine combined with training promotes changes in skeletal muscle. Equine Veterinary Journal, supplement 34: 269-274.
Rodgers, M.R. (2006). Effects of Oral Glucosamine and Chondroitin Sulfates Supplementation on Frequency of Intra-articular Therapy of the Horse Tarsus. International Journal of Applied Research in Veterinary Medicine, 4(2): 155-162.
Schuback, K., Essén-Gustavsson, B., & Persson, G.B. (2000). Effect of creatine supplementation on muscle metabolic response to a maximal treadmill exercise test in Standardbred horses. Equine Veterinary Journal, 32(6): 533-540.
Sengupta, K., Alluri, K.V., Satish, A.R., Mishra, S., Golakoti, T., Sarma, K.V.S., Dey, D., & Raychaudhuri, S.P. (2008). A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin® for treatment of osteoarthritis of the knee. Arthritis Research & Therapy, 10(4): R85.
Sinatra, S.T., Chopra, R.K., Jankowitz, S., Horohov, D.W., & Bhagavan, H.N. (2013). Coenzyme Q10 in Equine Serum: Response to Supplementation. Journal of Equine Veterinary Science, 33: 71-73.
Smith, H.J., Mukerji, P., & Tisdale, M.J. (2005). Attenuation of Proteasome-Induced Proteolysis in Skeletal Muscle by B-Hydroxy-B-Methylbutyrate in Cancer-Induced Muscle Loss. Cancer Research, 65(1): 277-283.
Stefanon, B., Bettini, C., Guggia, P. (2000). Administration of branched-chain amino acids to Standardbred horses in training. Journal of Equine Veterinary Science, 20(2): 115-119.
Steiss, J.E. (2002). Muscle disorders and rehabilitation in canine athletes. Veterinary Clinics of North America: Small Animal Practice, 32(1): 267-285.
Stephens, D.W., & Krebs, J.R. (1986). Foraging Theory. Princeton University Press, Chichester, UK.
Szcześniak, K.A., Ostaszewski, P., Fuller Jr, J.C., Ciecierska, A., & Sadkowski, T. (2015). Dietary supplementation of b-hydroxy-b-methylbutyrate in animals – a review. Journal of Animal Physiology and Animal Nutrition, 99: 405-417.
Tabor, G. (2021). Physiotherapy for neck pain in the horse. UK-Vet Equine, 5(1): 37-41.
Tanvir, E.M., Hossen, S., Hossain, F., Afroz, R., Hua Gan, S., Khalil, I., & Karim, N. (2017). Antioxidant Properties of Popular Turmeric (Curcuma longa) Varieties from Bangladesh. Journal of Food Quality, vol. 2017: Article ID 8471785.
Teixeira, F.A., Araujo, A.L., Ramalho, L.O., Adamkosky, M.S., Lacerda, T.F., & Coelho, C.S. (2016). Oral creatine supplementation on performance of Quarter Horses used in barrel racing. Journal of Animal Physiology and Animal Nutrition,100: 513-519.
Trottier, N.L., Nielsen, B.D., Lang, K.J., Ku, P.K., & Schott, H.C. (2002). Equine endurance exercise alters serum branched-chain amino acid and alanine concentrations. Equine Veterinary Journal, supplement 34: 168-172.
Turner, S.P., Hess, T.M., Treiber, K., Mello, E.B., Souza, B.G., & Almeida, F.Q. (2011). Comparison of Insulin Sensitivity of Horses Adapted to Different Exercise Intensities. Journal of Equine Veterinary Science, 31: 645-649.
Urschel, K.L., & McKenzie, E.C. (2021). Nutritional Influences on Skeletal Muscle and Muscular Disease. Veterinary Clinics of North America: Equine Practice, 37(1): 139-175.
Urschel, K.L., Geor, R.J., Waterfall, H.L., Shoveller, A.K., & McCutcheon, L.J. (2010). Effects of leucine or whey protein addition to an oral glucose solution on serum insulin, plasma glucose and plasma amino acid responses in horses at rest and following exercise. Equine Veterinary Journal, 42 (supplement 38): 347-354.
van Weeran, P.R., & Brama, P.A.J. (2001). Physiology and pathology of the equine joint. Pferdeheilkunde, 17: 307-318.
van Weeran, R. (2014). Joint physiology: responses to exercise and training. In: Hinchcliff, K.W., Kaneps, A.J., & Geor, R.J. (eds) Equine Sports Medicine and Surgery (2nd Ed.). Saunders Elsevier, London, UK.
Welch, C.A., Potter, G.D., Gibbs, P.G., & Eller, E.M. (2012). Plasma Concentration of Glucosamine and Chondroitin Sulfate in Horses after an Oral Dose. Journal of Equine Veterinary Science, 32: 60-64.
White, G.W., Wynn Jones, E., Harman, J., & Sanders, T. (1994). The efficacy of orally administered sulphated glycosaminoglycan in chemically induced equine synovitis and degenerative joint disease. Journal of Equine Veterinary Science, 14(7): 350-353.
Williams, C.A. (2008). Antioxidants & Their Application to Feeding Horses. Published on the internet: https://www.vin.com/apputil/content/defaultadv1.aspx?pId=11262&id=3865424. [Accessed 8 August 2021].


